| Original Article, Biomed Biopharm Res., 2023; 20(1):3-12 doi: 10.19277/bbr.20.1.304; PDF version [+]; Portuguese html [PT] |
Comparative view of reactive hyperemia perfusion changes in the upper-limb by laser Doppler flowmetry and optoacoustic tomography
Sérgio Faloni de Andrade ![]() , Tiago Granja
, Tiago Granja ![]() & Luís Monteiro Rodrigues
& Luís Monteiro Rodrigues ![]()
CBIOS - Center for Biosciences & Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisboa, Portugal
Abstract
Laser Doppler flowmetry (LDF) is one of the most used technologies to access human in vivo blood perfusion. However, its single point measurement capacity in a depth that likely is lower than 1 mm, are major criticisms that limit its utility. New image-based techniques such as Optoacoustic Tomography (OAT) allow the non-invasive observation of larger tissue areas with greater spatial resolution. In this study, we compared synchronized LDF and OAT data during a Post-Occlusion Reactive Hyperemia (PORH) maneuver in the upper limb (occlusion of the brachial artery). Measurements were obtained in the volar forearm (OAT and LDF) and in the fingertip (LDF). All procedures respected the principles of good clinical practices for human research purposes. Results confirm that LDF and OAT signals are significantly correlated at the superficial plexus. LDF does not detect deeper vascular structures of the skin but, even so, it is still very useful to access perfusion in areas with higher capillary density such as the fingertip.
Keywords: OAT, LDF, reactive hyperemia, PORH
To Cite: Faloni de Andrade, S., Granja, T., & Monteiro Rodrigues L. (2023) Comparative view of reactive hyperemia perfusion changes in the upper-limb by laser Doppler flowmetry and optoacoustic tomography and meta-analysis. Biomedical and Biopharmaceutical Research, 20(1), 3-12.
Correspondence to:
Received 10/03/2023; Accepted 14/04/2023
Introduction
The ability of tissues to control their own local blood flow in proportion to their metabolic needs is one of the fundamental principles of circulatory function (1). A clear example of this mechanism is observed in skeletal muscle during exercise, a situation in which blood flow can dramatically exceed resting values (2,3). Adequate hemodynamics depends on an integrated complex process involving multiple sensors and effectors (4) and this includes a permanent adaptation between macrocirculation and microcirculation (5). Disturbance in these basic mechanisms might over-compensate adaptation, leading to disease (6).
Microcirculatory impairment seems to be in the origin of well known conditions such as hypertension, stroke, peripheral artery disease, among others (7, 8). However, these events do not occur independently and, ultimately will affect all components of the cardiovascular system (4). This justifies the continued interest in cardiovascular physiology and in microcirculation specifically, to find better, more descriptive markers with clinical relevance, enabling early diagnostic and better disease prevention (9, 10).
The study of human microcirculation in vivo has been at the origin of a wide variety of non-invasive instruments designed to quantitatively describe the individual’s vascular status, primarily exploring skin microcirculation (11,12). Some recent reviews have been published on this subject, showing laser Doppler flowmetry (LDF) as one of the most referenced technologies accessing microcirculation function (13,14). However, this optical-based technology provides tissue perfusion only on a single-point acquisition area of the skin at fixed depth, depending on the frequency of the laser light (15,16). Currently, new technologies are able to associate image quantification with biological markers, providing a wider view of the vascular physiology. Among these technologies are confocal microscopy and optical coherence tomography or, more recently, of photo-acoustic tomography (OAT) (17-19). However high cost and complex operation limit their applicability.
In the present paper we explore the in vivo cardiovascular responses obtained after a supra-systolic reactive hyperemia (PORH) applied in the upper arm, registered by LDF and OAT. The goal was to identify the major differences between the two optical-based technologies and, at the same time, to contribute to better understand the mechanisms of microcirculation adaptation involved in this experimental maneuver.
Materials and Methods
Recruited Volunteers
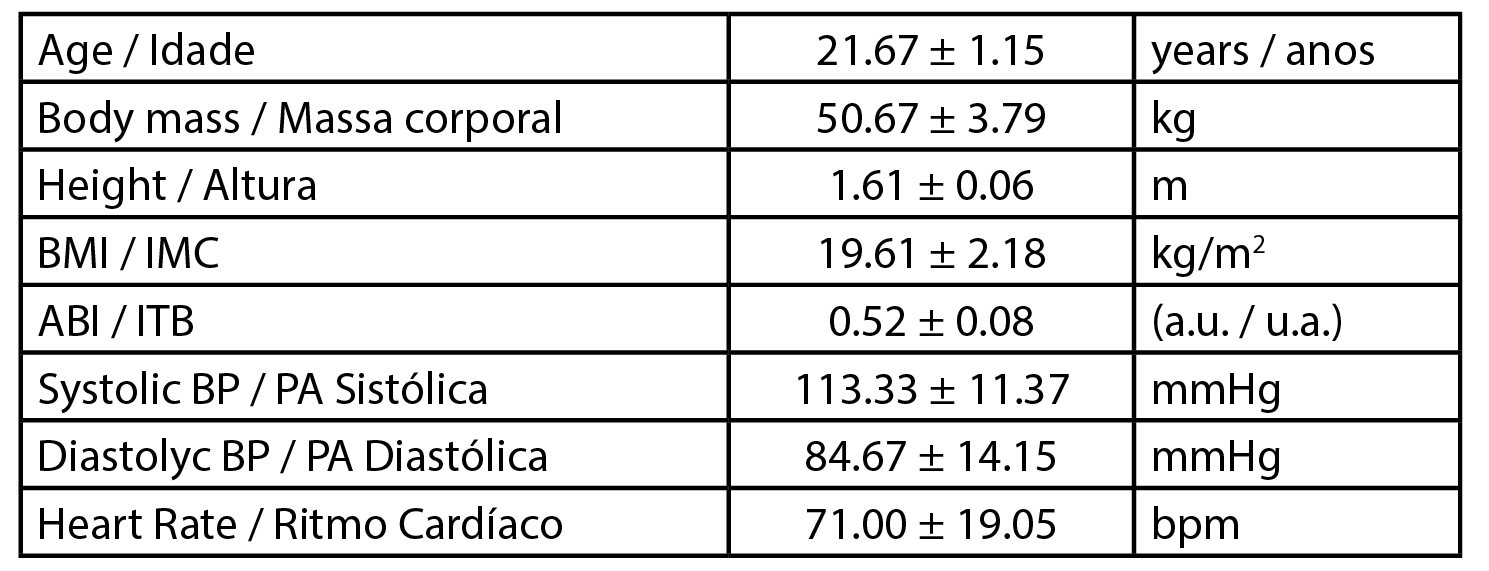
This work was developed with three female healthy participants (n=3) with a mean age of 21.67 ± 1.15 years, a mean body mass of 50.67 ± 3.79 kg and a mean body mass index (BMI) of 19.61 ± 2.18 kg/m2. Data related to the participants’ physiology are presented in Table 1. All procedures followed the principles of the Helsinki Declaration for good clinical practice and were previously approved by the Institutional Ethics Committee (Process CE.ECTS/P10.21). In brief, the selection of participants followed specific inclusion / non-inclusion criteria after informed written consent. Participants were non-smokers and free of any medication or food supplementation.
| Table 1 - Biometric characterization of volunteers. Body Mass Index (BMI), Ankle Brachial Index (ABI), arbitrary units (a.u.), Systolic blood pressure (Syst BP), Diastolic blood pressure (Diast BP), heart rate (HR). Values are expressed as Mean ± SD. |
 |
Experimental
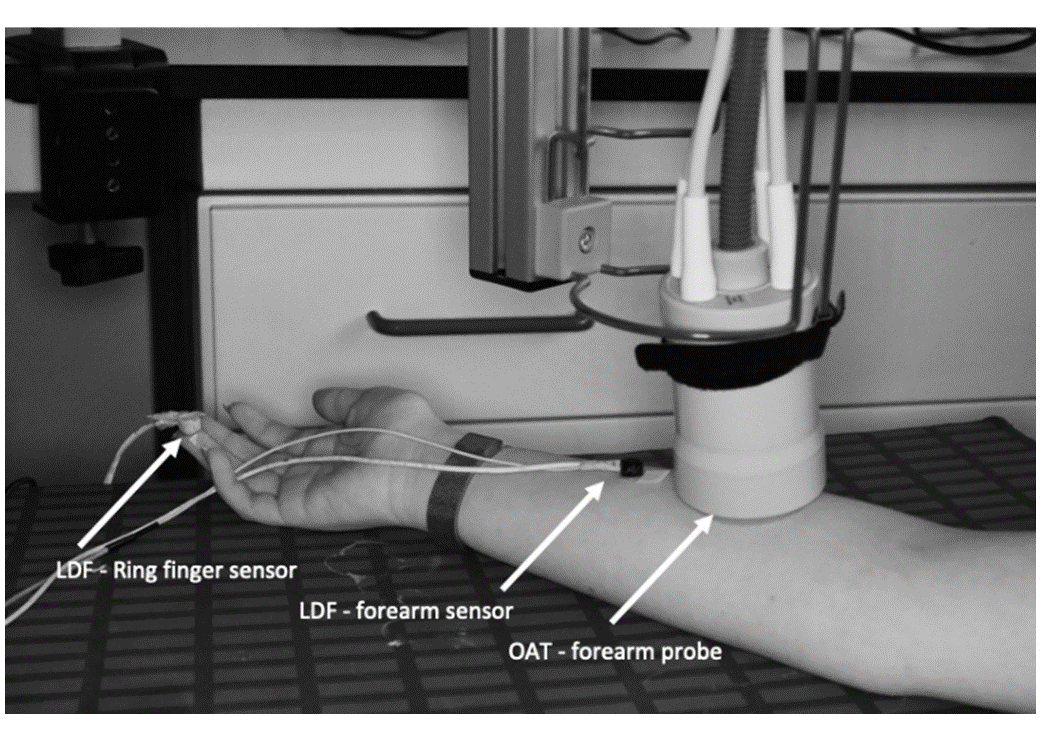
A post-occlusive reactive hyperemia (PORH) was used as a challenger. A pressure cuff was applied to the volunteers’ brachial upper arm area prior to the installation of LDF and OAT probes. Participants were brought to resting conditions accessed by heart rate and temperature stabilization to the laboratory environment. The OAT probe was placed at the ventral forearm area, previously described (15). Two LDF probes were equally placed on the same limb - one next to the OAT probe and the other LDF sensor fixed to the ventral aspect of the third finger on the same side (Figure 1). The PORH maneuver was recorded simultaneously in continuous mode in both OAT and LDF technologies over the course of four different stages, resting baseline, cuff inflation to 200 mmHg, occlusion, and recovery. The baseline was recorded for 1 min; the brachial cuff inflation to 200 mmHg was performed over 20 sec; occlusion was held for 1 min at 200 mmHg, and the post-occlusion recovery was recorded for 680 sec.
|
Figure 1 - LDF sensors and OAT probe setting prior to the PORH maneuverer. Two LDF sensors were placed at the volunteer’s ring finger (digit 4) fingertip and in the anterior middle - forearm. The OAT probe was stabilized proximal to the LDF anterior forearm sensor, fixed to a hinged support. The PORH manoeuvre was performed in the same arm where the LDF sensors and OAT probe were placed. |
 |
Perfusion was followed by PeriFlux System 5000 LDF (Perimed AB, Järfälla, Sweden), expressed in arbitrary units of perfusion (PU’s), using a red light with 780 nm and by OAT, using an Acuity MSOT (Multispectral Optoacoustic Tomography) imaging system (iThera Medical GmbH, Munich, Germany). The OAT system, which produces laser excitation pulses of 9 ns at wavelengths from 680 nm to 980 nm, acquired HbO2 and Hb chromophore spectra simultaneously for wavelengths ranging from 680 nm to 980 nm, projected as 3D images with a resolution depth of 1.5 cm3. Data reconstruction followed the manufacture protocol algorithms (viewMSOT 4.0) after ROI analysis of Hb and HbO2, allowing the calculation of total hemoglobin (HbT) and mean O2 saturation (MSOT SO2).
Data analysis
All graphical layouts were plot on GraphPad Prism 9.2.0 (283) MachineID: 0861F12DB8D10. Correlation analysis using Spearman’s two-tailed test were applied to all variables.
Results and Discussion
Exploratory tests such as the supra-systolic post-occlusive reactive hyperemia have been developed to assess local adaptive mechanisms to acute perfusion changes (17). Several proposed markers for microvascular disease (MVD) are related with the reactive perfusion response, however a wide variety of experimental conditions have been impaired proper data translation, limiting their utility (10,12,18). Nevertheless, mechanisms involved in microcirculatory adaptation to occlusion of a major vessel remains largely unknown. Recent results fail to support local reflex mechanisms (19,20) opening further research questions requiring interpretation.
Regarding assessment technologies, LDF provides information on blood perfusion velocity and volume at a relatively superficial depth (21,22). Considering the particular structure of skin microcirculation, we accept that LDF can access only a limited number of vessels and blood (13). Nevertheless, LDF has been extensively used as a gold standard to non-invasively monitor and evaluate local perfusion (23). Modern technologies such as OAT, with higher spatial and temporal resolutions, provide information on hemoglobin oxygenation (24-26). Spectroscopic and tomographic technologies have been applied to study the impact of PORH in skin microvasculature (27-30) but synchronization limitations related to peak recovery time persists (30). We have recently highlighted that microcirculatory HbO2 adjustment following PORH involves both micro and macrocirculation distributed at different tissue depths (23). Such reaction can only follow a centrally mediated response rather than a local reflex (4).
In the present study we explore the analytical performance of LDF and OAT under the same PORH experimental conditions (Figure 1 and Figure 2). Signals collected simultaneously with both systems were synchronized after data collection from all participants (Figure 3 and Figure 4).
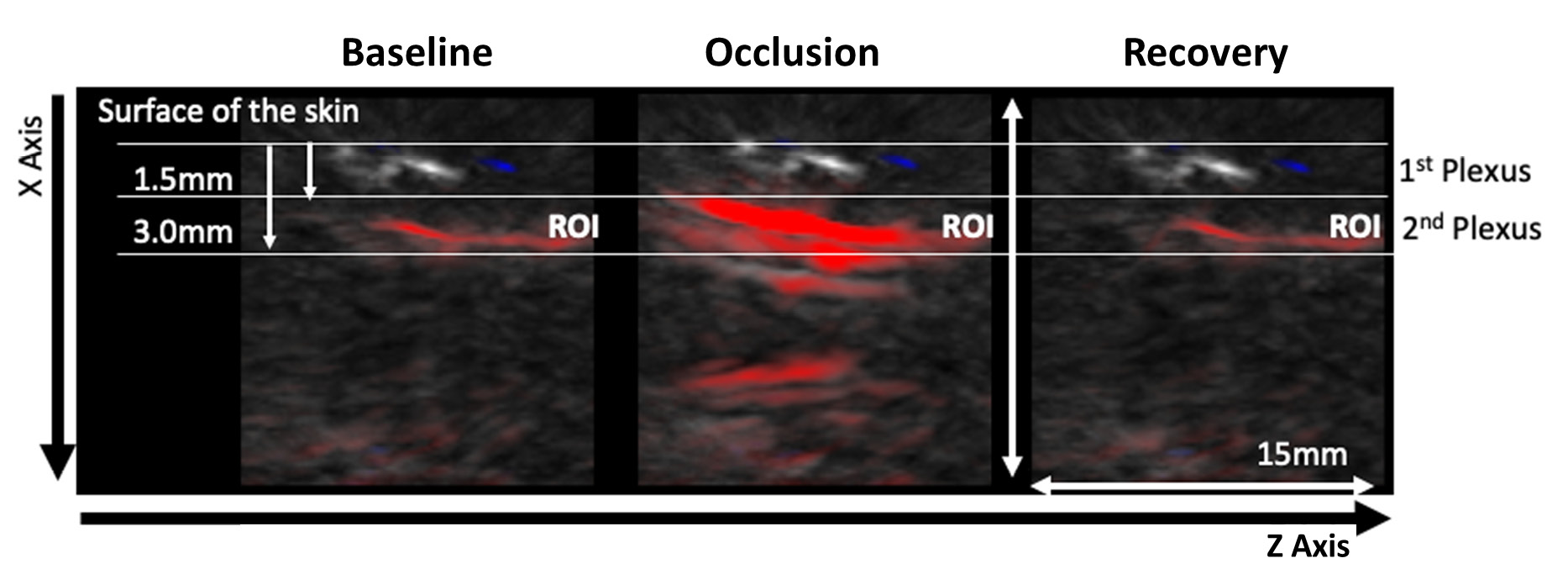
| Figure 2 - Representative OAT frames for data acquisition during the PORH protocol. The PORH maneuver involved three phases A) Baseline (60 sec); B) Occlusion at 200 mmHg (60 sec) with a brachial cuff; C) Post occlusive recovery (580 sec). Perfusion was recorded continuously. Videos of the skin microvasculature (axis XZ) show the impact of the challenger at the superficial plexus (1.5 mm deep) and at the deeper skin plexus (3.0 mm deep). The red colour represents the HbO2 chromophore. |
 |
|
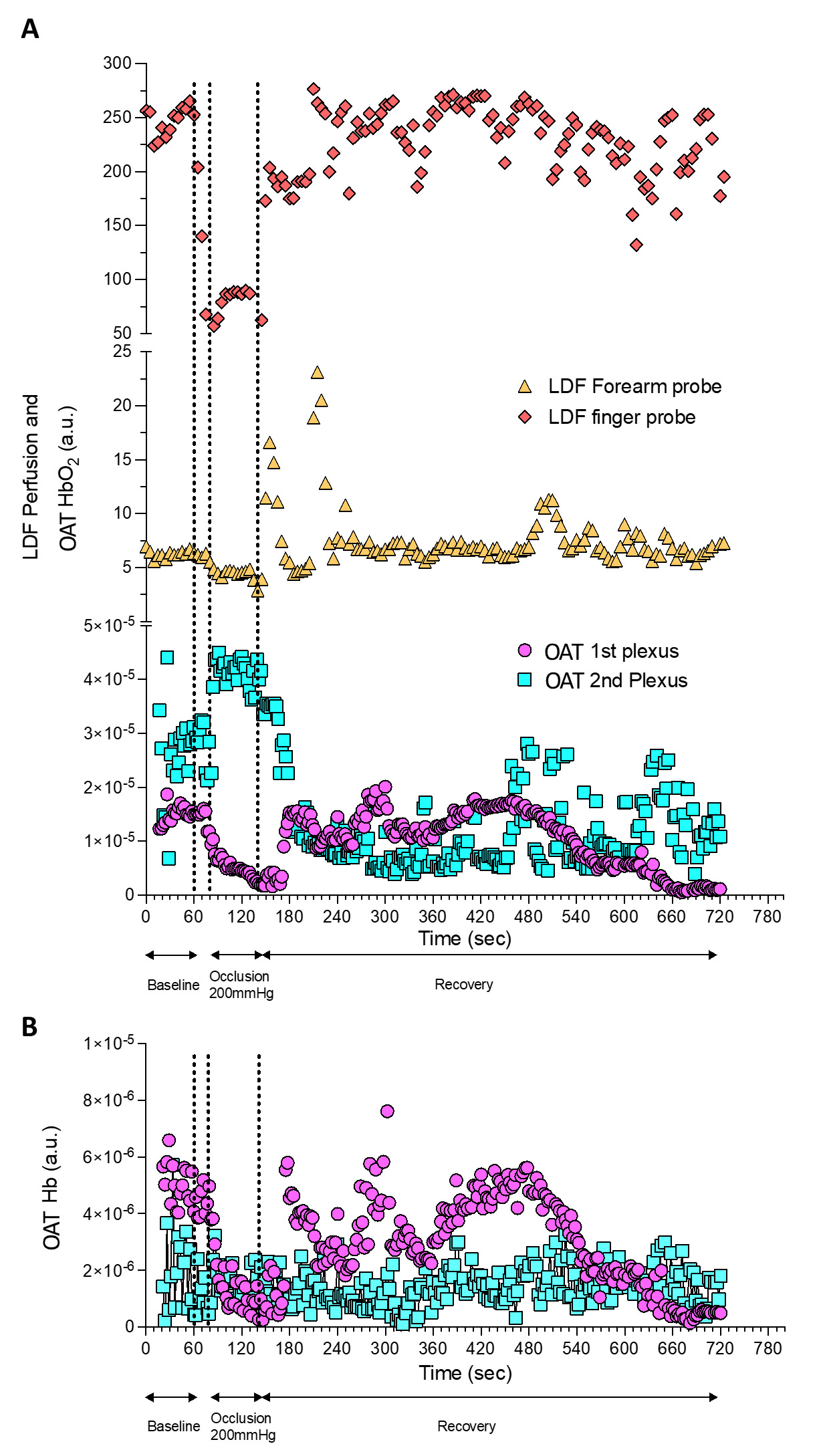
Figure 3 - Perfusion changes detected by OAT and LDF during a PORH maneuver. OAT and LDF acquired signals were continuously synchronized during the PORH protocol. |
 |
| Figure 4 - HbT and mSO2 calculated by OAT during a PORH maneuver. |
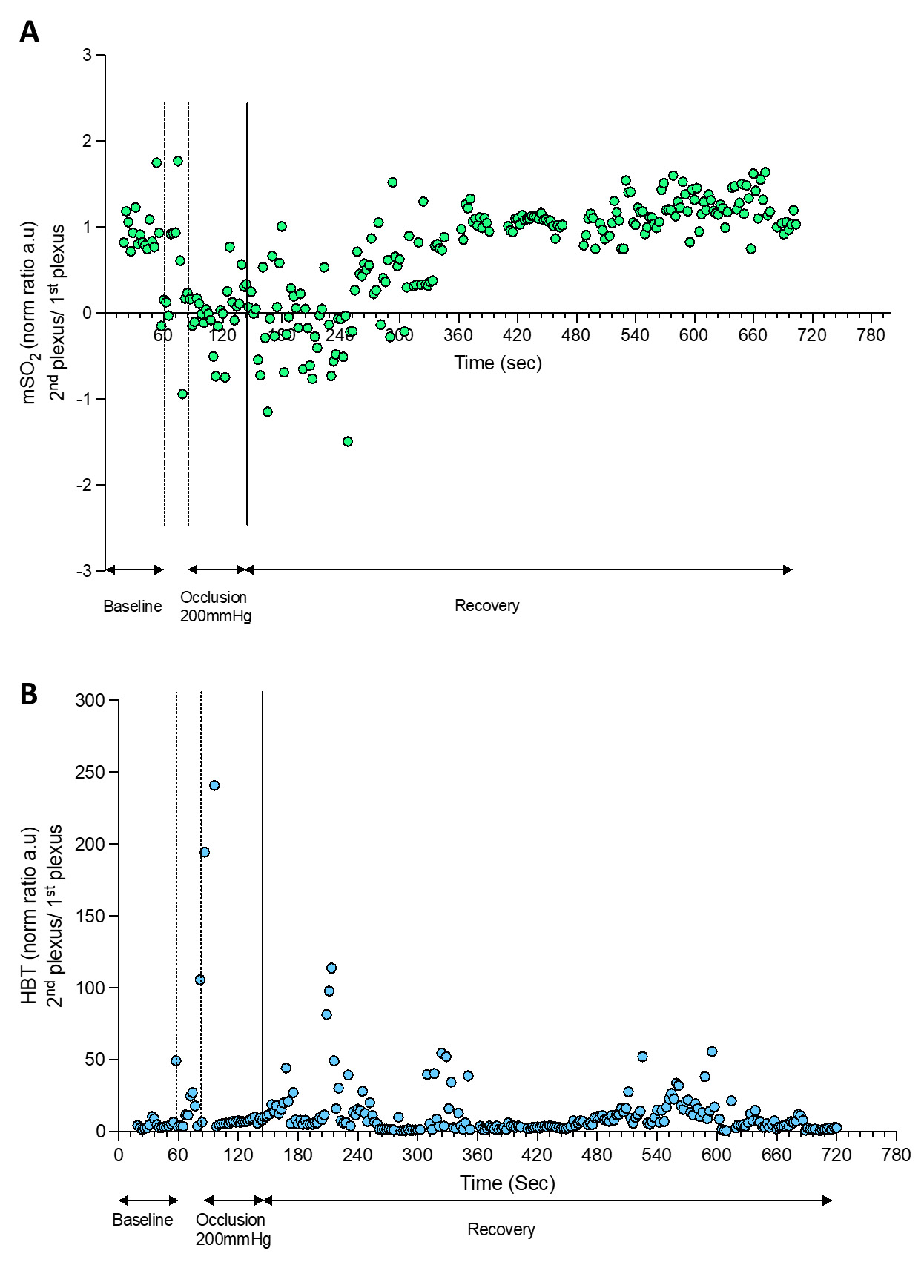 |
The present experimental conditions allowed us to obtain the same perfusion profile with LDF at the volar forearm and at the fingertip with the latter being more prominent (Figure 3A). This effect is possible due to the dense microcirculation network present at the skin of the fingertip, compared with the forearm. This allows higher Doppler effect on single-point measurements, compared at the same skin depth with the volar forearm (31-33). In fact, at the volar forearm skin, vascular structures are wider and the space between plexus longer, while they are much closer to each other at the fingertip (32).
The PORH protocol involved a rapid reduction of tissue perfusion with the cuff occlusion (never reaching “zero”), followed by a rapid increase of perfusion of HbO2 after the cuff deflation, reaching baseline values during recovery. OAT offers much different spatial and temporal resolutions compared to LDF in a volume of 1.5 mm3 (34). Therefore, through OAT we were able to identify both the superficial and the deeper skin plexus. Additionally, we can show that occlusion progressively provokes a HbO2 perfusion decrease at the superficial plexus and an increase of HbO2 at the deeper plexus, explained by blood transfer between plexus recently described (23,25).
The cuff deflation allowed recovery of HbO2, with restoration to baseline levels after 580 seconds. As perceived, the occlusion is taking place in the arm (brachial artery) and measurements are being obtained at the skin volar forearm and fingertip. Thus these responses are not local but rather mediated by a central / medullar reflex (4).
LDF and OAT technologies differ in terms of spatial resolution, tissue penetration depth, interaction between the light and the tissue and as a consequence, in the nature of the provided variables. Nevertheless, our correlation analysis found significant positive correlations between LDF signals registered in the fingertip and in the volar forearm, and between the fingertip LDF signal and the OAT superficial plexus signal.
In conclusion, this exploratory study confirms that both technologies have utility in measuring local as distal effects of forced circulatory changes, although much greater detail is available using OAT.
Funding
This research is funded by ALIES and COFAC, the principal providers of the OAT technology, and by Fundação para a Ciência e a Tecnologia (FCT) through the grant UIDB/04567/2020 to CBIOS.
Contribution of the authors
All authors equally contributed to the manuscript as it shows in its final form.
Conflict of Interests
The editors involved in this manuscript's authorship had no participation in the review or decision process. All authors have stated that there are no financial and/or personal relationships that could represent a potential conflict of interest.
References
1. Hall, J. E. (2020). Guyton and Hall Textbook of Medical Physiology (14th Edition ed.).
2. Segal, S. S. (1994). Cell-to-cell communication coordinates blood flow control. Hypertension, 23(6 Pt 2), 1113-1120. doi:10.1161/01.hyp.23.6.1113
3. Trinity, J. D., Broxterman, R. M., & Richardson, R. S. (2016). Regulation of exercise blood flow: Role of free radicals. Free radical biology & medicine, 98, 90-102. doi:10.1016/j.freeradbiomed.2016.01.017
4. Rodrigues, L. M., Rocha, C., Ferreira, H. T., & Silva, H. N. (2020). Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. Journal of Applied Physiology, 128(5), 1217-1226. doi:10.1152/japplphysiol.00437.2019
5. Climie, R. E., Gallo, A., Picone, D. S., Di Lascio, N., van Sloten, T. T., Guala, A., . . . Bruno, R. M. (2019). Measuring the Interaction Between the Macro- and Micro-Vasculature. Frontiers in Cardiovascular Medicine, 6. doi:10.3389/fcvm.2019.00169
6. Lemaster, K., Jackson, D., Goldman, D., & Frisbee, J. C. (2017). Insidious incrementalism: The silent failure of the microcirculation with increasing peripheral vascular disease risk. Microcirculation, 24(2), e12332. doi:https://doi.org/10.1111/micc.12332
7. SenthilKumar, G., Gutierrez-Huerta, C. A., Freed, J. K., Beyer, A. M., Fancher, I. S., & LeBlanc, A. J. (2022). New developments in translational microcirculatory research. American journal of physiology. Heart and circulatory physiology, 323(6), H1167-H1175. doi:10.1152/ajpheart.00566.2022
8. Chade, A. R. (2011). Renovascular disease, microcirculation, and the progression of renal injury: role of angiogenesis. American journal of physiology. Regulatory, integrative and comparative physiology, 300(4), R783-790. doi:10.1152/ajpregu.00657.2010
9. Clough G., & Cracowski, J.-L. (2012). Spotlight Issue: Microcirculation—From a Clinical Perspective. Microcirculation, 19(1), 1-4. doi:https://doi.org/10.1111/j.1549-8719.2011.00142.x
10. Glazkov, A. A., Krasulina, K. A., Glazkova, P. A., Kovaleva, Y. A., Bardeeva, J. N., & Kulikov, D. A. (2023). Skin microvascular reactivity in patients with diabetic retinopathy. Microvascular Research, 147, 104501. doi:10.1016/j.mvr.2023.104501
11. Abularrage, C. J., Sidawy, A. N., Aidinian, G., Singh, N., Weiswasser, J. M., & Arora, S. (2005). Evaluation of the microcirculation in vascular disease. Journal of vascular surgery, 42(3), 574-581. doi:10.1016/j.jvs.2005.05.019
12. Virdis, A., & Taddei, S. (2011). How to evaluate microvascular organ damage in hypertension: assessment of endothelial function. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension, 18(4), 163–167. https://doi.org/10.2165/11593630-000000000-00000.
13. Humeau, A., Steenbergen, W., Nilsson, H., & Strömberg, T. (2007). Laser Doppler perfusion monitoring and imaging: novel approaches. Medical & Biological Engineering & Computing, 45(5), 421-435. doi:10.1007/s11517-007-0170-5
14. Lal, C., & Leahy, M. J. (2016). An Updated Review of Methods and Advancements in Microvascular Blood Flow Imaging. Microcirculation, 23(5), 345-363. doi:https://doi.org/10.1111/micc.12284
15. Rajan, V., Varghese, B., van Leeuwen, T. G., & Steenbergen, W. (2009). Review of methodological developments in laser Doppler flowmetry. Lasers in medical science, 24(2), 269-283. doi:10.1007/s10103-007-0524-0
16. Fagrell, B., & Nilsson, G. (1995). Advantages and Limitations of One-Point Laser Doppler Perfusion Monitoring in Clinical Practice. Vascular Medicine Review, vmr-6(2), 97-101. doi:10.1177/1358863x9500600202
17. Roustit, M., & Cracowski, J. L. (2012). Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation, 19(1), 47-64. doi:10.1111/j.1549-8719.2011.00129.x
18. Yang, J., Zhang, G., Chang, W., Chi, Z., Shang, Q., Wu, M., . . . Jiang, H. (2020). Photoacoustic imaging of hemodynamic changes in forearm skeletal muscle during cuff occlusion. Biomedical optics express, 11(8), 4560–4570. https://doi.org/10.1364/BOE.392221
19. Rosenberry, R., & Nelson, M. D. (2020). Reactive hyperemia: a review of methods, mechanisms, and considerations. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 318(3), R605-R618. doi:10.1152/ajpregu.00339.2019
20. Young, G. M., Krastins, D., Chang, D., Lam, J., Quah, J., Stanton, T., . . . Askew, C. D. (2021). The Association Between Contrast-Enhanced Ultrasound and Near-Infrared Spectroscopy-Derived Measures of Calf Muscle Microvascular Responsiveness in Older Adults. Heart, Lung and Circulation, 30(11), 1726-1733. doi:https://doi.org/10.1016/j.hlc.2021.07.004
21. Bonner, R. F., & Nossal, R. (1990). Principles of Laser-Doppler Flowmetry. In A. P. Shepherd & P. Å. Öberg (Eds.), Laser-Doppler Blood Flowmetry (pp. 17-45). Boston, MA: Springer US.
22. Rajan, V., Varghese, B., van Leeuwen, T. G., & Steenbergen, W. (2009). Review of methodological developments in laser Doppler flowmetry. Lasers in Medical Science, 24(2), 269-283. doi:10.1007/s10103-007-0524-0
23. Monteiro Rodrigues, L., Granja, T. F., & de Andrade, S. F. (2022). Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology. Life, 12(10), 1628. Retrieved from https://www.mdpi.com/2075-1729/12/10/1628
24. Hacker, L., Brunker, J., Smith, E. S. J., Quiros-Gonzalez, I., & Bohndiek, S. E. (2020). Photoacoustics resolves species-specific differences in hemoglobin concentration and oxygenation. Journal of biomedical optics, 25(9), 095002. https://doi.org/10.1117/1.JBO.25.9.095002
25. Granja, T., Faloni de Andrade, S., & Rodrigues, L. M. (2022). Multispectral Optoacoustic Tomography for Functional Imaging in Vascular Research. Journal of visualized experiments : JoVE,(184), 10.3791/63883. doi:10.3791/63883
26. Karlas, A., Fasoula, N.-A., Katsouli, N., Kallmayer, M., Sieber, S., Schmidt, S., . . . Ntziachristos, V. (2023). Skeletal muscle optoacoustics reveals patterns of circulatory function and oxygen metabolism during exercise. Photoacoustics, 30, 100468. doi:https://doi.org/10.1016/j.pacs.2023.100468
27. Dennis, J. J., Wiggins, C. C., Smith, J. R., Isautier, J. M. J., Johnson, B. D., Joyner, M. J., & Cross, T. J. (2021). Measurement of muscle blood flow and O2 uptake via near-infrared spectroscopy using a novel occlusion protocol. Scientific Reports, 11(1), 918. doi:10.1038/s41598-020-79741-w
28. McLay, K. M., Nederveen, J. P., Pogliaghi, S., Paterson, D. H., & Murias, J. M. (2016). Repeatability of vascular responsiveness measures derived from near-infrared spectroscopy. Physiological Reports, 4(9), e12772. doi:https://doi.org/10.14814/phy2.12772
29. Yang, J., Zhang, G., Chang, W., Chi, Z., Shang, Q., Wu, M., . . . Jiang, H. (2020). Photoacoustic imaging of hemodynamic changes in forearm skeletal muscle during cuff occlusion. Biomedical Optics Express, 11(8), 4560-4570. doi:10.1364/BOE.392221
30. Didier, K. D., Hammer, S. M., Alexander, A. M., Caldwell, J. T., Sutterfield, S. L., Smith, J. R., . . . Barstow, T. J. (2020). Microvascular blood flow during vascular occlusion tests assessed by diffuse correlation spectroscopy. Experimental Physiology, 105(1), 201-210. doi:https://doi.org/10.1113/EP087866
31. Tibiriçá, E., Matheus, A. S. M., Nunes, B., Sperandei, S., & Gomes, M. B. (2011). Repeatability of the evaluation of systemic microvascular endothelial function using laser doppler perfusion monitoring: clinical and statistical implications. Clinics, 66(4), 599-605. doi:https://doi.org/10.1590/S1807-59322011000400013
32. Roustit, M., Blaise, S., Millet, C., & Cracowski, J. L. (2010). Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvascular Research, 79(2), 102-108. doi:https://doi.org/10.1016/j.mvr.2010.01.001
33. Pouratian, N., & Toga, A. W. (2002). 5 - Optical Imaging Based on Intrinsic Signals. In A. W. Toga & J. C. Mazziotta (Eds.), Brain Mapping: The Methods (Second Edition) (pp. 97-140). San Diego: Academic Press.
34. Tiago Granja, S. F. d. A., and Luis Monteiro Rodrigues. (2021). Optoaccoustic Tomography – good news for microcirculatory research. Biomedical and Biopharmaceutical Research, 18(2), 200-212. doi: 10.19277/bbr.18.2.269
