| Original Article, Biomed Biopharm Res., 2022; 19(1):114-132 doi: 10.19277/bbr.19.1.277; pdf version [+] ; Portuguese html version [PT] |
Reporting suspected adverse reactions to COVID-19 vaccines in Portugal: side effects per vaccine type, gender, and age up to July 2021
Carla Pires
CBIOS – Universidade Lusófona’s Research Center for Biosciences & Health Technologies, Campo Grande 376, 1749-024 Lisboa, Portugal.
corresponding author:
Abstract
Adverse drug reactions (ADRs) of COVID-2019 vaccines require close monitoring. This study aimed to describe and discuss the most frequent ADRs of COVID-2019 vaccines and their prevalence per age group, gender, and vaccine type in Portugal up to 22 July 2021. ADR reports of COVID-19 vaccines were collected from a public report of INFARMED, I.P. (the Portuguese Medicines Agency). There were 11,314 ADRs notified per 11,002,983 COVID-19 vaccines administered in Portugal – 1 ADR per 1,000 vaccines administered and 0.4 serious ADR per 1,000 vaccines administered. The most commonly reported ADRs were myalgia, headache, pyrexia, pain at the injection site, and fatigue. More ADRs occurred in women and younger individuals. A slightly higher percentage of reported ADRs occurred with Vaxzervria. In conclusion, ADRs were mainly related to non-serious reactions. COVID-19 vaccines maintain a favourable safety profile in Portugal, although the types and prevalence of ADRs are likely to differ between different types of COVID-19 vaccines. Men may need to be motivated to more frequently report ADRs.
Keywords: COVID-19; adverse drug reactions; vaccination; COVID-19 vaccines; pharmacovigilance
Received: 08/02/2022; Accepted: 20/03/2022
Introduction
Severe acute respiratory syndrome-2 virus (SARS-CoV-2) is a contagious respiratory virus, which is the causative agent of the 2019 coronavirus disease (COVID-19). COVID-19 was first reported in Wuhan, China in December 2019, with a cluster of viral pneumonia cases being reported to the World Health Organization (WHO) on 31 December 2019. SARS-CoV-2 quickly spread all over the world, with the WHO declaring the current pandemic on 11 March 2020 (1).
An unprecedented global effort was taken by the scientific community to develop vaccines against COVID-19, since vaccination was seen as the hope to build group protection, reduce disease spread and mitigate serious forms of COVID-19 (2). Impressively, diverse stakeholders publicly announced new COVID-19 vaccines in the late trimester of 2020, for example:
- 9 November 2020, Pfizer-BioNTech announced via a press release that the vaccine efficacy of BNT162b2 was greater than 90%;
- 16 November 2020, Moderna reported a 94% vaccine efficacy of mRNA-1273 via a press release;
- 8 December 2020, AstraZeneca/Oxford University reported interim results, with the viral-vectored ChAdOx1 showing an efficacy of 70% across two schedules; and
- 29 January 2021, the Johnson & Johnson (Janssen) COVID-19 single-shot vaccine showed 66% efficacy overall (1).
In the European Union (EU), there were four authorized vaccines against COVID-19 with conditional marketing authorizations granted by the European Medicine Agency (EMA) in July 2021: two mRNA-based vaccines – Comirnaty, BNT162b2 (Pfizer-BioNTech) (date of issue of marketing authorization valid throughout the EU 21/12/2020; International non-proprietary name (INN): COVID-19 mRNA vaccine (nucleoside-modified); tozinameran) and Spikevax, mRNA-1273 (Moderna) (date of issue of marketing authorization valid throughout the EU 06/01/2021; INN: COVID-19 mRNA vaccine, nucleoside modified), and two viral-vector based vaccines: Vaxzevria, AZD1222 (AstraZeneca) (date of issue of marketing authorization valid throughout the EU 29/01/2021; INN: COVID-19 vaccine (ChAdOx1-S [recombinant]) and COVID-19 Vaccine Janssen, Ad26.COV2.S (Janssen-Cilag) (date of issue of marketing authorization valid throughout the EU 11/03/2021; INN: COVID-19 vaccine (Ad26.COV2-S [recombinant]) (3, 4). Importantly, a 7.6% reduction in the case fatality ratio, 95% confidence interval (CI) = -12.6% to -2.7% (p = 0.002), was achieved, with a 10% increase in vaccine coverage (data from 90 countries over 25 weeks: November 2020 to April 2021) (5).
Globally, the supply and full coverage of COVID-19 vaccines is essential to control the pandemic, which is expected to occur during 2023. Several efforts are being taken by WHO, in collaboration with various countries, to ensure an equitable access to vaccines against COVID-19, although more than 80% of doses were administered to people in high-income and upper-middle-income countries through July 2021 (6). Lessons from the COVID-19 pandemic should be taken into consideration during the evaluation of future pandemic scenarios, such as protecting groups at greater risk, saving the majority of lives, and/or ensuring societal benefit, especially since the development of new vaccines may not be achieved so quickly in the case of a new pandemic virus (7).
SARS-CoV-2 is likely to become an endemic virus, and the production, distribution, supply, and administration of COVID-19 vaccines, including the update of these vaccines against possible new variants, remains an important topic. Governments and health authorities are responsible for an ethical and equitable management of the stocks of COVID-19 vaccines (7-9). It is possible that “the need for large-scale vaccination program will be transient until an endemic state for SARS-CoV-2 is reached” (9).
Possible impact of age and gender on the ADR profiles of COVID-19 vaccines
In general, adverse reactions to Comirnaty (Pfizer-BioNTech) vaccine, to Vaxzevria (AstraZeneca) COVID-19 vaccine, and to Spikevax (Moderna) COVID-19 vaccine were milder and were reported less frequently in older adults (65 years and older) than in younger people (10). However, serious ADRs, such as death, permanent disability, and hospitalization were more frequent in older adults in another study (11). For instance, reports of adverse drug reactions were significantly associated with age group, with a higher occurrence in the group ≤45 years (n = 409 healthcare personnel; AstraZeneca (AZ), Pfizer-BioNTech (PB), and SinoPharm (SP)* vaccines (12).
*A SinoPharm (SP) is not authorised for use in the European Union.
Women tend to be more prone to have more adverse events following vaccination in comparison to men, because women usually produce immunity responses, such as higher antibodies levels (13-14). In a questionnaire-based survey (German Healthcare Workers, February–March 2021; n = 599), the local and systemic side effects were not statistically significant between women and men in the case of mRNA-based vaccines: Comirnaty (BNT162b2, Pfizer-BioNTech) and Spikevax (mRNA-1273, Moderna) vaccine, but statistically significant differences were found between women and men for local and systemic side effects in the case of the viral vector-based vaccine Vaxzevria (AstraZeneca), with women reporting a higher proportion of side effects (15). In opposition to these findings, gender was not identified as a significant risk factor of AstraZeneca COVID-19 vaccine side effects among European healthcare workers during February–March 2021 (U = 680; p = 0.539) (16). Age and gender did not significantly affect the duration and severity of adverse events in a study of 1,736 individuals who had received a first or second dose of the vaccine (Pfizer-BioNTech, AstraZeneca or AstraZeneca-Oxford, and Sinopharm) at least 30 days before the survey (17).
ADRs per type of COVID-19 vaccine
mRNA vaccines present a safe profile and a limited number of side effects. Common side effects included heat, pain, swelling and erythema at the injection site, fever and chills, fatigue, headaches, decreased appetite, myalgia (muscle pain), arthralgia (joint pain). Serious adverse reactions included anaphylaxis/anaphylactic shock and Bell’s palsy (18-20). Among the reported local and systemic side effects of the AstraZeneca COVID-19 vaccine were injection site pain, injection site swelling, injection site redness, fatigue, headache, nausea, feeling unwell, myalgia, arthralgia, fever, chills, or lymphadenopathy. In addition, oral and skin-related side effects were also reported, such as, ulcers/blisters/vesicles, white/red plaque, halitosis, bleeding gingiva, swollen lips, taste alterations, or skin rash (16). Similar to other COVID-19 vaccines, local reactions (e.g., pain, erythema, or swelling) and/or systemic reactions (e.g., headache, fatigue, myalgia, nausea, or fever) were also reported with the single-dose Janssen Ad26.COV2.S vaccine against COVID-19 (21).
Moreover, different COVID-19 vaccines may differ in terms of ADR frequency and/or type. For example, the incidence of adverse drug reactions was higher among recipients after one dose of AZD1222 (Vaxzevria, AstraZeneca) than in those with the first and the second dose of the BNT162b2 vaccine (Comirnaty, Pfizer-BioNTech) (80 and 1,440 healthcare workers who received two doses of BNT162b2 and one dose of the AZD1222 vaccines, respectively) (22). A recent review pointed out that the “pooled rates of local and systemic reactions were significantly lower among inactivated vaccines (23.7%, 21.0%), protein subunit vaccines (33.0%, 22.3%), and DNA vaccines (39.5%, 29.3%), compared to RNA vaccines (89.4%, 83.3%), non-replicating vector vaccines (55.9%, 66.3%), and virus-like particle vaccines (100.0%, 78.9%)”. The frequency of serious adverse events was low for all studied vaccines (<0.1%) (23).
Thus, the aim of this study was to describe and discuss the safety data of COVID-19 vaccines in Portugal (cumulative occurrences until 22 July 2021), including the most reported ADRs and the profile of ADRs per vaccine type, gender and age.
Materials and Methods
Definition of adverse reaction
Adverse reaction synonyms used in this paper: Adverse drug reaction (ADR), adverse event or side effect. An ADR is a “response to a medicinal product which is noxious and unintended [DIR 2001/83/EC Art 1(11)]” (24). Side effects, also known as adverse events, are unwanted or unexpected events or reactions to a drug (25).
An adverse event following immunization is defined as “any untoward medical event that follows immunization and that does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom, or disease” (26).
Data sources
Data were collected from two recent publications of INFARMED, I.P. (the Portuguese Medicine Agency), which correspond to the data from the same report, regarding the safety of COVID-19 vaccines in Portugal up to 22 July 2021. These reports are public, and aggregate data from all reported ADR, which are potentially related to COVID-19 vaccines up to 22 July 2021 within Portugal (27-28).
Self-reporting of ADRs
The reporting of a suspected side effect(s) per se does not reflect any confirmation of a potential link between the medicinal product/vaccine and the observed effect(s) and should not be used to determine the likelihood of a certain side effect. The causal relationship between taking a medicine and an ADR is established by specialists from different expertise areas through the application of both qualitative and quantitative techniques. Thus, it is possible to determine if an ADR is consequence of taking a certain medicine or if it is a mere coincidence (27-28, 30-31). All suspected ADRs may be reported online by health professionals or patients/citizens in Portugal (https://www.infarmed.pt/web/infarmed/submissaoram). Only four elements are necessary to produce a valid Individual Case Safety Report (ICSR): “one identifiable reporter, one single identifiable patient, at least one suspect adverse reaction, and at least one suspect medicinal product”. After receipt and validation, the information is evaluated by a team of experts (pharmacists and physicians). Finally, the fully anonymized information is sent to European and worldwide ADR databases, Eudravigilance, and Vigibase, respectively, for the purpose of a more comprehensive and continuous evaluation of the medicinal product's safety profile (29). According to the INFARMED, I.P.'s report, it is likely that some patients have opted not to report the suspected ADR, because of the lack of severity and/or if the ADR was already known (27-28).
Serious ADRs were categorised according to the WHO classification as follows: persistent or significant disability/incapacity; congenital anomaly; life threatening, death or other clinically relevant. ADRs are described by the subject who reports the ADR: a healthcare professional and/or a citizen/patient, and if at least one ADR is classified as serious, the case is also classified as serious (26-28).
COVID-19 vaccines
The COVID-19 vaccines administered in Portugal up to July 2021 were Comirnaty (BNT162b2, Pfizer-BioNTech) and Spikevax (mRNA-1273, Moderna) (both mRNA-based vaccines), along with Vaxzevria, (AZD1222, AstraZeneca) and the Janssen COVID-19 vaccine (Ad26.COV2.S, Janssen-Cilag) (viral-vector-based vaccines). These vaccines are also used in EU (3, 4).
Results
Up to 22 July 2021, the number of cumulative administered vaccines was 11,002,983. According to the findings of the last national census (2021), there are 9,860,175 inhabitants in Portugal (4,684,642 men and 5,175,533 women) (32).
Reported ADRs
The cumulative number of reported ADRs was 11,314 (100%) up to 22 July 2021 (36% serious and 64% non-serious): 1 ADR per 1,000 administered vaccines in Portugal, and 0.4 serious ADRs per 1,000 administered vaccines in Portugal (27-28). From the n=11,314 (100%) reported ADRs, the 15 most reported ADRs are presented in Table 1. The three most frequent ADRs were myalgia, headache, and pyrexia, with similar percentages (Table 1).
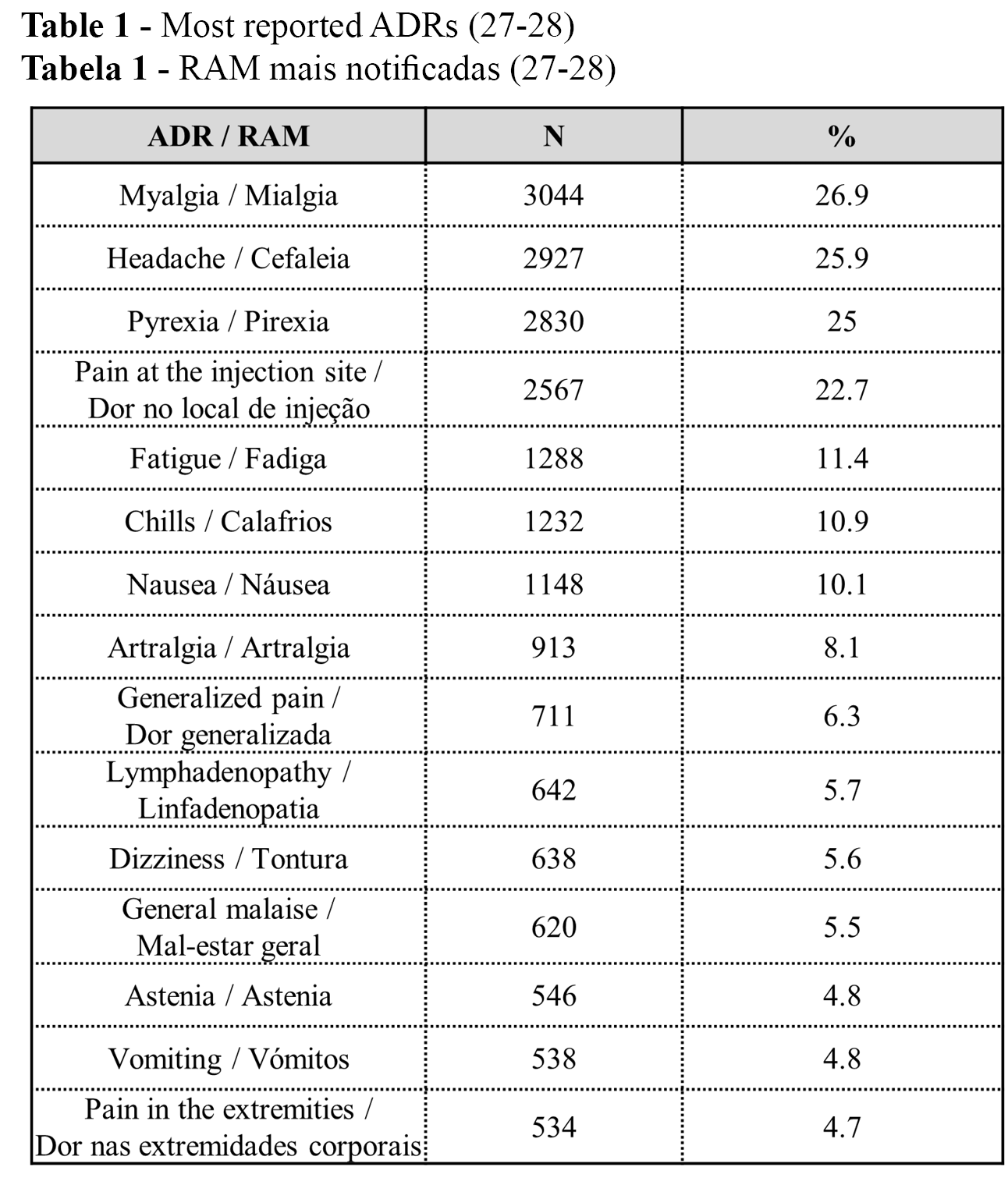
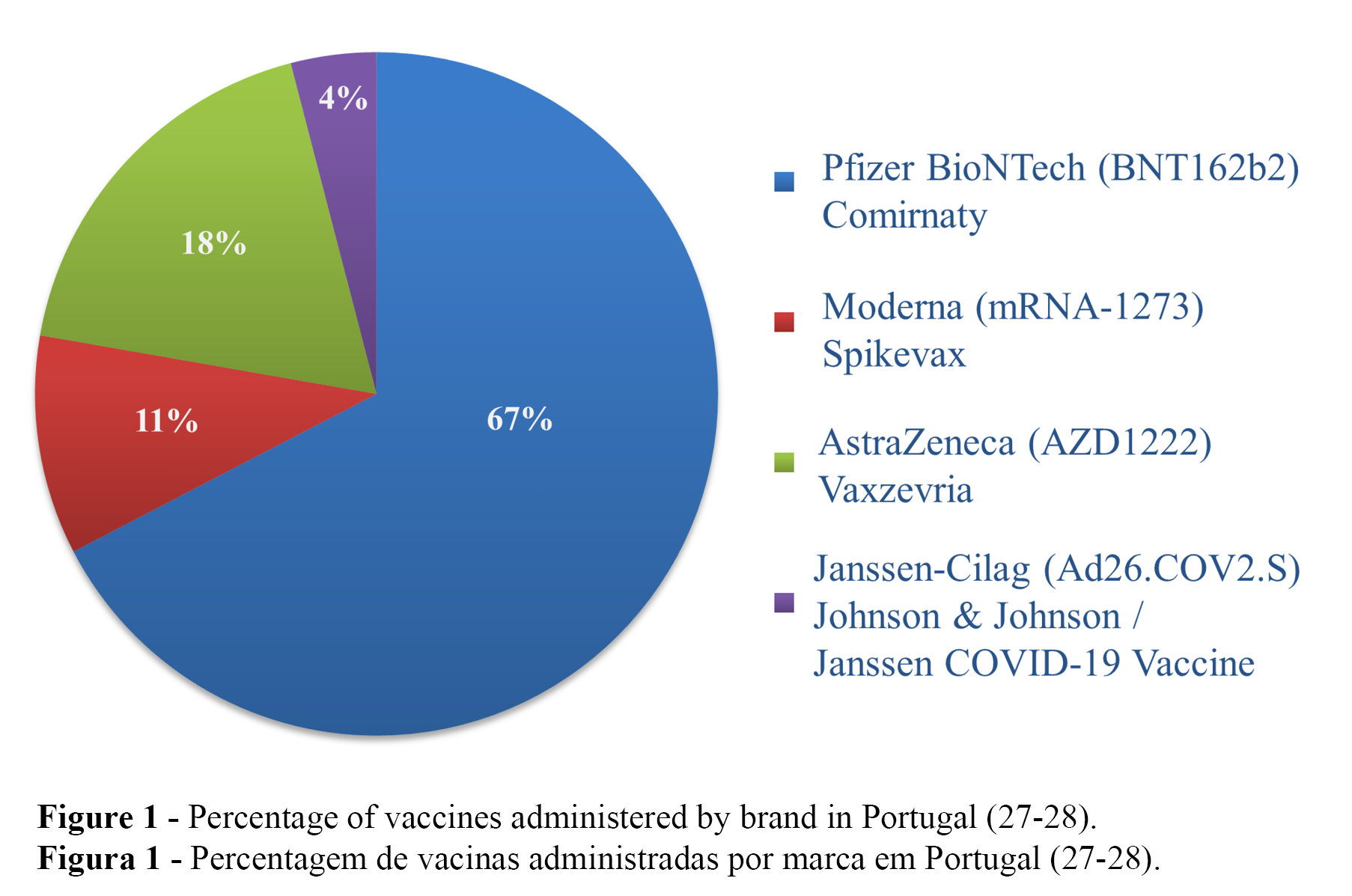
Administered vaccines and ADRs per vaccine type
Of the 11,002,983 (100%) administered vaccines in Portugal, 67.4% (n = 7,412,497) were Comirnaty (Pfizer-BioNTech), 18.2% (n = 2,003,932) were Vaxzevria (AstraZeneca), 10.4% (n = 1,141,821) were Spikevax (Moderna), and 4.0% (n = 444,733) were the Janssen COVID-19 Vaccine (Janssen-Cilag) (Figure 1) (27-28). The number of reported ADRs per vaccine type up to 22 July 2021 were as follows: Comirnaty (Pfizer-BioNTech) (n = 6,485), Spikevax (Moderna) (n = 970), Vaxzevria (AstraZeneca) (n = 3,480), and COVID-19 Vaccine Janssen (Janssen-Cilag) (n = 379), with the highest number of reported ADRs per 1,000 administered vaccines for Vaxzevria (AstraZeneca) (1.7), followed by Comirnaty (Pfizer-BioNTech) and COVID-19 Vaccine Janssen (Janssen-Cilag), with equal values (0.9), and the lowest value for Spikevax (Moderna) (0.8) (28).
ADRs per gender
The reporting of suspected ADRs was more prevalent in women (n = 7,800, 68.9%) than men (n = 2,615, 23.1%). The sex was classified as unknown in 7.9% of ICSR (n = 899) (27-28).
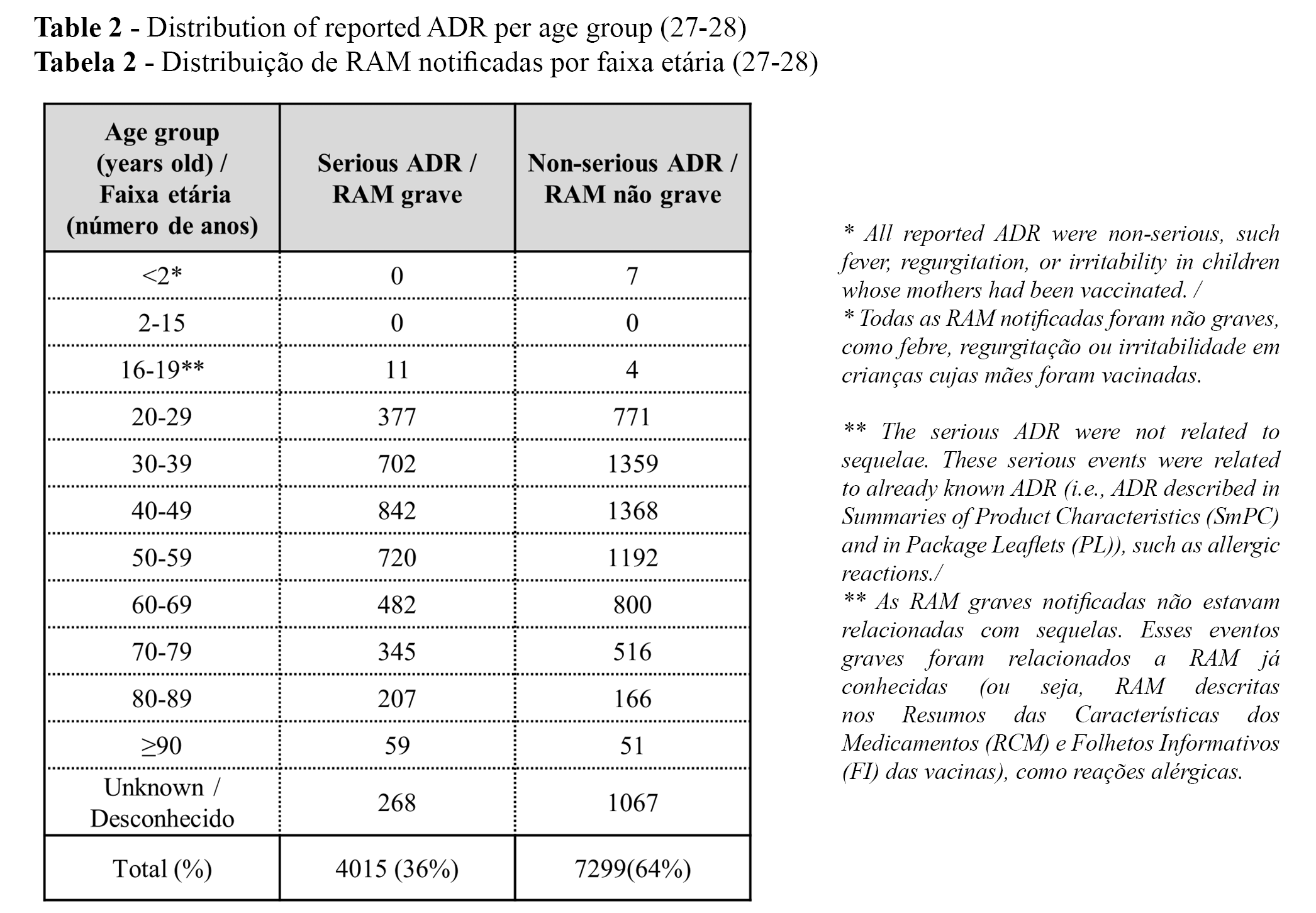
ADRs per age group and at least one dose of COVID-19 vaccine
The distribution of the reported ADRs per age group is presented in Table 2. The distribution of the reported ADR (serious vs. non-serious and total) per age group (up to 22 July 2021) and at least one dose of COVID-2019 vaccine (up to 25 July 2021)1 is presented in Table 3. (27-28).
1DGS – Direcção Geral de Saúde. Portuguese Report of Vaccination. Week 29 (27/12/2020 to 25/07/2021). Available online: https://static-storage.dnoticias.pt/www-assets.dnoticias.pt/documents/Relatorio_Vacinacao_Week29.pdf
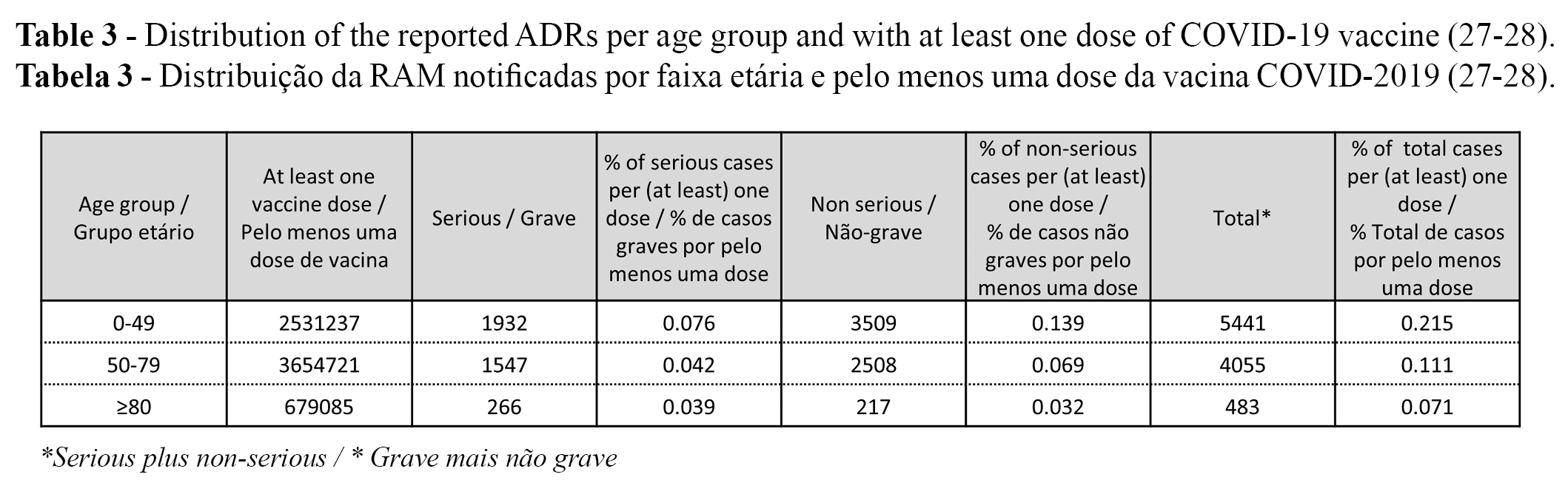
The percentage (serious, non-serious and total) of reported ADRs with at least one dose of COVID-19 vaccine decreased per age group (i.e., % of ADRs per age groups: 0-49 > 50-79 ≥ 80). The highest % was found in the group of 0-49 years old, followed by the groups of 50-79 years old and ≥80 years old, respectively (Table 3). It should be considered that ADRs were reported up to 22 July 2021, but the number of vaccinated people with at least one dose was only identified on 25 July 2021 (Table 3).
Discussion
The most reported ADRs
Among the most frequently reported ADRs of COVID-19 vaccines have been pain, swelling, fever, fatigue, chills, myalgia, arthralgia, headache, itching, and redness (33-35). Other local and/or systemic ADRs were also reported, such as vomiting, nausea, or dizziness (34-35). Thus, the most reported ADRs in the present study seems to be congruent with those reported in the literature, which contributes to supporting the validity of the data from the present study.
According to INFARMED, I.P., the most reported ADRs were within the reactogenic profile of any vaccine. These ADRs were identified during clinical trials of COVID-19 vaccines and are included in Summaries of Product Characteristics (SmPC) and in Package Leaflets (PL). Approximately 15.3 million COVID-19 vaccine doses were given in Australia up to 15 August 2021: the most frequent side effects included injection-site reactions, headache, myalgia, fever, and chills, which are among the most reported side effects in Portugal up to 22 July 2021 (33).
In general, the discomfort associated with these ADRs was resolved in a few hours/days, without the need of medical intervention and without (further) sequelae (27-28, 33). A study including 599 German healthcare workers reported that the majority (84.9%) of side effects resolved within 1–3 days after vaccination: Comirnaty (Pfizer-BioNTech) and Spikevax (Moderna) vaccine (mRNA-based vaccines; n = 474), and 125 Vaxzevria (AstraZeneca) (viral vector-based vaccine) (36).
ADRs per age group and with at least one dose of COVID-19 vaccine
The highest percentage of notified ADRs was found in the 0-49 year old group, followed by the groups of 50-79 years old and ≥80 years old, for both serious and non-serious ADRs re(Table 3). The differences between age groups may be explained because younger adults are more prone to have ADRs than older individuals (e.g., due to a higher immunologic response) or because younger adults are more accustomed to reporting ADRs online.
A higher prevalence of ADRs with COVID-19 vaccines in younger receipients is also reported in official publications (10). In a study describing the characteristics of ADR reports following COVID-19 vaccination (n = 8,976) with the mRNA vaccines authorized by the Food and Drug Administration (FDA) in the USA, younger adults (18 to 64 years) reported more adverse events following COVID-19 vaccination compared to older adults, but the latter were more likely to report serious adverse events, such as death, permanent disability, and hospitalization (14 December 2020 to 22 January 2021) (11). A significant increased risk of side effects was identified in the younger age group (≤39 years old) with mRNA-based or viral vector-based vaccines (599 healthcare professionals) (15).
Gender
There were more suspected ADRs from COVID-19 vaccines in women (68.9%) than in men (23.1%), which follows the general ADR reporting profile for all medicinal products in Portugal, which may be explained by the following facts: women may give more attention to health issues than men (27-28) or women may be more prone to develop ADRs than men (13-14). It did not seem appropriate to identify possible differences between ADRs of COVID-19 vaccines per gender using only the present data, therefore additional clinical studies are recommended. These findings are aligned with the data from the EudraVigilance database on 5 September 2021: COVID-19 mRNA vaccine Spikevax (Moderna) (CX-024414) (74,306, 69.4% women; 31,782, 29.7% male; 972, 0.9% not specified; total 107,060, 100%); COVID-19 mRNA vaccine Comirnaty (Pfizer-BioNTech; Tozinameran) (290,099, 72.3% women; 105,137, 26.2% male; 6,264, 1.6% not specified; total 401,500, 100%); COVID-19 vaccine Vaxzevria (AstraZeneca) (ChAdOx1 nCOV-19) (263,671, 71.8% women; 94,407, 25.7% male; 9,046, 2.5% not specified; total 367,124, 100%); and COVID-19 vaccine Janssen (Janssen-Cilag) (AD26.COV2.S) (15,415, 59.6% women; 10,107, 39.1% male; 328, 1.3% not specified; total 25,850, 100%) (31). Men may need to be motivated to more frequently report ADRs of COVID-19 vaccines in the EU, including Portugal.
Gender may be correlated with the prevalence of certain ADRs per COVID-19 vaccine. For instance, women may develop more ADRs than men, because they produce a higher immune response than men (11, 13, 15). In opposition to these findings, men were more likely to report serious adverse events, death, and hospitalization compared to women in a study with the two mRNA COVID-19 vaccines that received emergency authorization in the USA (14 December 2020 to 22 January 2021) (11). Thus, more studies are recommended regarding this topic (e.g., additional tailored clinical studies), namely sex and gender differences should be evaluated in the clinical trials of vaccines against COVID-19 (37).
Additionally, a questionnaire on ADRs may be provided to all subjects in the days following COVID-19 vaccination, or social studies may be implemented to better understand the differences between women and men on the profile of ADRs and the motivations to report ADRs. Preferably, these questionnaires should also be paper based, because some citizens have limited digital skills. A voluntary, smartphone-based safety surveillance system was developed by the Centers for Disease Control and Prevention (CDC) to provide information on adverse reactions after COVID-19 vaccination. The platform was effective, since voluntary reports to v-safe did not find unexpected patterns of adverse reactions after an additional dose of COVID-19 vaccine (n=12,591 participants). (38).
Administered vaccines and ADRs per vaccine type
The most administered vaccine in Portugal was Comirnaty (Pfizer-BioNTech), representing around 70% of all administered vaccines up to 22-7-2021 (27-28). A similar percentage was achieved in EU, with Comirnaty (Pfizer-BioNTech) representing 72% of all administered doses of COVID-2019 vaccines in EU up to 28 April 2021 (39). This similar profile/proportion of administration of Comirnaty may be justified by similar vaccination policies within the EU. Among other the EU Vaccines Strategy aims to “ensure the quality, safety and efficacy of vaccines”, “to secure timely access to vaccines for Member States and their population while leading the global solidarity effort”, “to ensure equitable and affordable access for all in the EU to an affordable vaccine as early as possible”, and “to make sure that preparations are made in EU countries regarding the roll-out of safe and effective vaccines, addressing transportation and deployment needs, and identifying priority groups.” Additionally, the European Commission decided to implement an advanced purchase agreements on COVID-19 vaccines and a centralized approach on procuring COVID-19 vaccines on behalf of the Member States (40).
Overall, Portugal had 1 only ADR per 1,000 administered vaccines from the 11,314 reported ADRs (100%) up to 22 July 2021 (27-28). On 30 September 2021, there were 1.2 reported ADRs per 1000 administered COVID-2019 vaccines in EU (41). These findings are indicative of a similar and low ADR reporting rate in Portugal and EU. In contrast, Australia comparatively registered 3.3 ADRs per 1,000 administered vaccines from the 50,597 reported ADRs (100%) up to 15 August 2021 (33). These differences may be due to a lower ADR reporting rate in Portugal/EU than in Australia, and/or the types of COVID-19 vaccines administered. In Portugal, Comirnaty (Pfizer-BioNTech), Spikevax (Moderna), Janssen COVID-19 Vaccine (Janssen-Cilag), and Vaxzevria (AstraZeneca) vaccines were administered. In Australia, Comirnaty (Pfizer-BioNTech) and Vaxzevria (AstraZeneca) vaccines were administered. COVID-19 vaccines ADRs may be under-reported to medicines agencies (42). Thus, post-vaccination surveys on vaccine efficacy and safety and more post-vaccination studies regarding safety and efficacy are recommended to all recipients. Post-vaccination surveys may be a more appropriate tool to monitor the safety of COVID-19 vaccines than the usual pharmacovigilance reporting systems. For instance, these questionnaires may be delivered by health authorities through national websites of vaccination certificates.
In the present study, Comirnaty (Pfizer-BioNTech), Spikevax (Moderna) (mRNA vaccines), and COVID-19 Vaccine Janssen (Janssen-Cilag) (adenovirus vector vaccine) presented a similar number of notified ADRs per 1,000 administered vaccines, while Vaxzevria (AstraZeneca) (adenovirus vector vaccine) presented a slightly higher number of reported ADRs per 1,000 administered vaccines (27-28, 43). These findings are supported by other studies, with Vaxzevria (AstraZeneca)/AZD1222 presenting more reported ADRs than Comirnaty (Pfizer-BioNTech)/BNT162b2. Of 1,520 health workers, 80 received 2 doses of BNT162b2 vaccine and 1,440 received a first dose of AZD1222 vaccine: 52.5% and 76.2% of subjects reported adverse events after the first and second dose of BNT162b2, respectively, and 90.9% subjects reported adverse events after the first dose of AZD1222 (p < 0.001) (22).
In addition, in the EU up to 28 April 2021, the COVID-19 vaccines with more reported ADRs were Vaxzervria (0.67%; 184833 ADRs per 27430533 doses), followed by the Janssen COVID-19 vaccine (0.42%; 413 ADRs per 98139 doses), COVID-19 vaccine Spikevax (Moderna) (0.18%; 17625 ADRs per 9691295 doses), and Comirnaty (Pfizer-BioNTech) (0.16%; 151306 ADRs per 96519666 doses) (27-28, 31, 39). The differences between the Portuguese and EU profiles of reported ADRs per vaccine type may be explained by the fact that ADR reporting in Portugal was based on 11,002,983 (100%) administered vaccines up to 22 July 2021, and ADRs reported in the EU were based on 133,739,633 (100%) administered vaccines up to 28 April 2021, i.e., findings based on the administration of a higher number of vaccines may be more sensitive. Moreover, cultural differences or the knowledge on how to report ADRs by country may explain the differences between the profiles of ADR reporting.
Globally, Vaxzevria (AstraZeneca) seems to be associated with a slightly higher number of ADRs (27-28, 39). Some citizens may have been more predisposed to report suspected ADRs related to Vaxzevria (AstraZeneca) or Janssen COVID-19 vaccine as a result of the occurrence of rare serious thrombotic events that were largely communicated by mass media and medicines agencies (44-46).
Study limitations
The causes of subjects´ vaccination hesitancy and/or the psychosocial predictors of willingness to receive a COVID-19 vaccine were not specifically evaluated in the present study. However, on 22 October 2021 Portugal was the second most vaccinated country globally, which is demonstrative of a limited vaccination hesitancy in this territory (47). According to a study from the European Commission on the attitudes on vaccination against COVID-19 in the EU, Portugal achieved the highest rates on (believing) the benefits of the COVID-19 vaccine outweigh the risks (87%) and on the “civic duty” of vaccination (86%). Further, the vast majority of Portuguese (82%) were satisfied with the management of the vaccination strategy by the Government and 89% agreed that the EU played a key role in ensuring access to COVID-19 vaccines in Portugal (48). Altogether, these findings may explain the low vaccine hesitancy regarding COVID-2019 vaccination in Portugal.
The self-reporting of ADRs by vaccinated citizens may be related to some inconsistencies/errors, since symptoms or health issues may be due to causes other than vaccination itself, or individuals may be psychologically worried by taking a COVID-19 vaccine and, consequently, motivated to report any symptom.
At least to some extent, younger subjects may have self-reported more ADRs because of their better proficiency regarding digital skills. Citizens with limited health literacy and fewer digital skills are more likely to experience more difficulties in reporting ADRs online. Self-reporting of ADRs per vaccine type may have been influenced/enhanced by some news of COVID-19 vaccines in social media (e.g., thrombotic events after Vaxzevria/AstraZeneca vaccination).
Conclusion
The benefits of COVID-19 vaccination far outweigh the potential risks of vaccination in Portugal (27-28, 33). Overall, the proportion of reported ADRs (serious and non-serious) was limited (1 ADR per 1,000 administered COVID-19 vaccines and 0.4 serious ADRs per 1,000 administered COVID-19 vaccines up to 22 July 2021), with a confirmed favourable vaccine safety profile (27-28). However, ADRs may have been under reported in Portugal, since higher self-reporting rates were comparatively reported in other countries (e.g., Australia) (33).
The reporting of suspected ADRs was mainly related to non-serious common reactions (e.g., swelling, fever, fatigue, chills, myalgia and pain at the administration site), which follows the normal patterns of reported ADRs in clinical trials, vaccines package leaflets, and/or vaccines ADR reports from other countries (33-35). These side effects usually resolved, without any consequence or hospitalization, in a few hours/days (27-28, 33, 39).
The highest percentage of reported of notified ADRs was found in the youngest age group, which is in line with the findings of other studies (11-12, 15, 49). Further studies (e.g., clinical studies) are recommended to check these potential differences in incidence, prevalence, and type of ADR per gender or age group (e.g., profile of ADRs per age group) (37).
Of the four administered COVID-19 vaccines in Portugal: Vaxzervria (AstraZeneca), COVID-19 Vaccine Janssen (Janssen-Cilag), Spikevax (Moderna), and Comirnaty (Pfizer-BioNTech) at the date of the present study, it seems that Vaxzervria (AstraZeneca) presents a slightly higher percentage of reported ADRs than the remaining vaccines. These data are supported by other studies and data from ADR databases (22, 31, 39). ADRs were more prevalent in women than men, which follows the general reporting tendency in Portugal and in the EU. This may be because women give more attention to health issues than men (27-28, 49). In this sense, men may need to be motivated to more regularly report ADRs.
Practical implications and future research
The exclusive use of self-reporting may not be the best method to monitor ADRs to COVID-19 vaccines, since subjects may underreport ADRs (e.g., lack of digital skills, lack of knowledge on how to report, or because the ADR is non-serious or the ADR is known or expected). In contrast, subjects may be influenced to report several kinds of ADRs (e.g., possible impact of social media news on the number and type of COVID-19 vaccines reporting of suspected ADRs). Overall, more studies are needed to confirm these suppositions, such as research to evaluate possible cultural differences on the profile and prevalence of self-reporting ADRs between different countries or the distribution of questionnaires regarding the occurrence of ADRs in the days after COVID-19 vaccination. The design and implementation of social studies (e.g., questionnaires) is highly recommended to better understand why certain populational groups (e.g., women) may self-report more ADRs of COVID-19 vaccines than other groups (e.g., men) or to minimise a possible under reporting of suspected ADRs.
Additionally, more clinical studies may be required to accurately study the profile of ADRs per COVID-19 vaccine or to determine possible links between age or gender and ADR profile, since subjects are required to regularly consult a healthcare professional and perform clinical analyses during clinical studies. The prevalence of side effects per type of ADR and/or vaccine should also be analyzed, for example, mRNA-based vaccines seem to be associated with a higher prevalence of local side effects (e.g., injection site pain), and viral vector-based vaccines seem to be associated with a higher prevalence of systemic adverse reactions (e.g., headache / fatigue) (15).
Authors Contributions Statement
The conceptualization, data analysis, writing (draft and original version), and review of the paper were carried out by Carla Pires. The author has read and agreed to the published version of the manuscript.
Funding
None.
Acknowledgements
The author acknowledges CBIOS - Research Center for Biosciences and Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Lisbon, Portugal.
Conflict of Interests
The author declares there are no financial and/or personal relationships that could present a potential conflict of interests.
Reference
- Carvalho, T., Krammer, F., & Iwasaki, A. (2021). The first 12 months of COVID-19: a timeline of immunological insights. Nature reviews. Immunology, 21, 245–256.
- Pormohammad, A., Zarei, M., Ghorbani, S., Mohammadi, M., Razizadeh, M.H., Turner, D.L., et al. (2021). Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines, 9, 467.
- EMA. COVID-19 vaccines: authorized, 2021. Disponível online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section(Acedido em 5-9-2021).
- Francis, A. I., Ghany, S., Gilkes, T., & Umakanthan, S. (2021). Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgraduate medical journal, 140654. https://doi.org/10.1136/postgradmedj-2021-140654.
- Liang, L. L., Kuo, H. S., Ho, H. J., & Wu, C. Y. (2021). COVID-19 vaccinations are associated with reduced fatality rates: Evidence from cross-county quasi-experiments. Journal of global health, 11, 05019.
- Padma, T.V. (2021). COVID vaccines to reach poorest countries in 2023 — despite recent pledges. Nature, 595, 342-343.
- Williams, J., Degeling, C., McVernon, J., & Dawson, A. (2021). How should we conduct pandemic vaccination? Vaccine, 39, 994–999.
- Frederiksen, L., Zhang, Y., Foged, C., & Thakur, A. (2020). The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Frontiers in immunology, 11, 1817.
- Veldhoen, M., & Simas, J.P. (2021). Endemic SARS-CoV-2 will maintain post-pandemic immunity. Nat Rev Immunol, 21, 131–132.
- Medicines & Healthcare products Regulatory Agency. Research and analysis Coronavirus vaccine - weekly summary of Yellow Card reporting, Updated 7 October 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- Xiong, X., Yuan, J., Li, M., Jiang, B., & Lu, Z.K. (2021). Age and Gender Disparities in Adverse Events Following COVID-19 Vaccination: Real-World Evidence Based on Big Data for Risk Management. Frontiers in medicine, 8, 700014.
- Abu-Hammad, O., Alduraidi, H., Abu-Hammad, S., Alnazzawi, A., Babkair, H., Abu-Hammad, A., et al. (2021). Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines, 9, 577.
- Migliore, L., Nicolì, V., & Stoccoro, A. (2021). Gender Specific Differences in Disease Susceptibility: The Role of Epigenetics. Biomedicines, 9, 652.
- Ciarambino, T., Barbagelata, E., Corbi, G., Ambrosino, I., Politi, C., Lavalle, F., Ruggieri, A., & Moretti, A. (2021). Gender differences in vaccine therapy: where are we in COVID-19 pandemic? Monaldi archives for chest disease.https://doi.org/10.4081/monaldi.2021.1669.
- Klugar, M., Riad, A., Mekhemar, M., Conrad, J., Buchbender, M., Howaldt, H. P., et al. (2021). Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers.Biology, 10, 752.
- Riad, A., Pokorná, A., Mekhemar, M., Conrad, J., Klugarová, J., Koščík, M., et al. (2021). Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines,9, 673.
- Qutaiba A. Al Khames Aga, Waseem H., et al. (2021). Safety of COVID-19 vaccines. J Med Virol., 1–7. https://doi.org/10.1002/jmv.27214.
- Anand, P., & Stahel, V. P. (2021). Review the safety of Covid-19 mRNA vaccines: a review. Patient safety in surgery, 15, 20.
- Baden, L.R., El Sahly, H.M., Essink, B., Kotloff, K., Frey, S., Novak, R., et al. (2021). Efficacy and Safety of the mRNA–1273 SARS-CoV–2 Vaccine. N Engl J Med., 384, 403–16.
- Polack, F.P., Thomas, S.J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. (2020). Safety and Efficacy of the BNT162b2 mRNA Covid–19 Vaccine. N Engl J Med., 383, 2603–15.
- Sadoff, J., Gray, G., Vandebosch, A., Cárdenas, V., Shukarev, G., Grinsztejn, B., Spiessens, B., et al. (2021). ENSEMBLE Study Group Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med., 384, 2187–2201.
- Wi, Y.M., Kim, S.H., & Peck, K.R. (2021). Early Adverse Events between mRNA and Adenovirus-Vectored COVID-19 Vaccines in Healthcare Workers. Vaccines, 9, 931.
- Wu, Q., Dudley, M. Z., Chen, X., Bai, X., Dong, K., Zhuang, T., Salmon, & D Yu, H. (2021). Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC medicine, 19, 173.
- HMA & EMA. (2017). Guideline on good pharmacovigilance practices (GVP). Disponível online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf(Acedido em 5-9-2021).
- FDA. (2018). Finding and Learning about Side Effects (adverse reactions). Disponível online: https://www.fda.gov/drugs/information-consumers-and-patients-drugs/finding-and-learning-about-side-effects-adverse-reactions(Acedido em 5-9-2021).
- WHO. (2020). COVID-19 Vaccines: Safety Surveillance Manual. Disponível online: https://www.who.int/vaccine_safety/committee/Module_AEFI.pdf?ua=1 (Acedido em 4-9-2021).
- INFARMED, I.P. (2021). Pharmacovigilance Report – Monitorization of the safety of COVID-2019 vaccines [Relatório de Farmacovigilância Monitorização da segurança das vacinas contra a COVID-2019 em Portugal), received data until 22-7-2021. Disponível online: https://www.infarmed.pt/web/infarmed/infarmed/-/journal_content/56/15786/4562594(Acedido em 4-9-2021).
- INFARMED, I.P. (2021). Pharmacovigilance Bulletin. 25(5). Disponível online: https://www.infarmed.pt/documents/15786/4230446/Boletim+de+Farmacovigil%C3%A2ncia%2C+Volume+25%2C+n%C2%BA5%2C+maio+de+2021/fb014726-c395-b78b-d75d-34c0c37939d5?version=1.0(Acedido em 23-10-2021).
- Brüssow, H. (2021). COVID-19: vaccination problems. Environmental microbiology, 23, 2878–2890.
- EMA. (2014). Recommendation on harmonizing the approach to causality assessment for adverse events to veterinary medicinal products (revision 1). Disponível online: https://www.ema.europa.eu/en/veterinary-regulatory/post-authorisation/pharmacovigilance/guidance/recommendation-harmonising-approach-causality-assessment-adverse-events-veterinary-medicinal(Acedido em 12-9-2021).
- EMA. (2021). EudraVigilance: European database of suspected adverse drug reaction reports.Disponível online: https://www.adrreports.eu/ (Acedido em 23-10-2021).
- INE – Instituto Nacional de Estatística. (2021). Censos 2021: resultados preliminares. Disponível online: https://ine.pt/scripts/db_censos_2021.html(Acedido em 5-9-2021).
- Therapeutic Goods Administration. (2021). COVID-19 vaccine weekly safety report - 19-08-2021. Disponível online: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-19-08-2021(Acedido em 12-9-2021).
- Cai, C., Peng, Y., Shen, E., Huang, Q., Chen, Y., Liu, P, et al. (2021). A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Molecular therapy : the journal of the American Society of Gene Therapy, 29, 2794–2805.
- Yuan, P., Ai, P., Liu, Y., Ai, Z., Wang, Y., Cao, W., et al. (2020). Safety, Tolerability, and Immunogenicity of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. medRxiv: the preprint server for health sciences, 2020.11.03.20224998. https://doi.org/10.1101/2020.11.03.20224998
- DGS – Direção Geral de Saúde. (2021). Portuguese COVID-2019 report on 4-9-2021. Disponível online: https://covid19.min-saude.pt/wp-content/uploads/2021/09/551_DGS_boletim_20210904.pdf(Acedido em 5-9-2021).
- Brady, E., Nielsen, M. W., Andersen, J. P., & Oertelt-Prigione, (2021). S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nature communications, 12, 4015.
- Hause, A. M., Baggs, J. Gee, J., Marquez, P., Myers, T. R., Shimabukuro, T. T., & Shay, D. K. (2021). Safety Monitoring of an Additional Dose of COVID-19 Vaccine - United States, August 12-September 19, 2021. MMWR. Morbidity and mortality weekly report, 70, 1379–1384.
- ECDC - European Centre for Disease Prevention and Control. (2021). Suspected adverse reactions to COVID19 vaccination and the safety of substances of human origin, June 2021. Disponível online: https://www.ecdc.europa.eu/sites/default/files/documents/Suspected-adverse-reactions-to-COVID-19-vaccination-and-safety-of-SoHO.pdf(Acedido em 23-10-2021).
- European Commission. (2021). EU Vaccines Strategy, 2021. Disponível online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/eu-vaccines-strategy_en(Acedido em 23-10-2021).
- European Medicine Agency. (2021). Safety of COVID-19 vaccines. Disponível online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/safety-covid-19-vaccines(Acedido em 23-10-2021).
- Jęśkowiak, I., Wiatrak, B., Grosman-Dziewiszek, P., & Szeląg, (2021). A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines, 9, 502.
- WHO. (2021). Statement for healthcare professionals: How COVID-19 vaccines are regulated for safety and effectiveness, 11 June 2021. Disponível online: https://www.who.int/news/item/11-06-2021-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness(Acedido em 5-9-2021).
- EMA. (2021). COVID-19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets, April 2021. Disponível online: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood(Acedido em 10-9-2021).
- Thakur, K.T., Tamborska, A., Wood, G.K., McNeill, E., Roh, D., & Akpan, I.J., et al. (2021). Clinical review of cerebral venous thrombosis in the context of COVID-19 vaccinations: Evaluation, management, and scientific questions. Journal of the neurological sciences, 427, 117532.
- Wise, J. (2021). Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ (Clinical research ed.), 372, n699.
- Our World in data. (2021). Statistics and Research: Coronavirus (COVID-19) Vaccinations, 2021.Disponível online: https://ourworldindata.org/covid-vaccinations(Acedido em 5-9-2021).
- European Union.(2021). Attitudes on vaccination against Covid-19, June 2021. Disponível online: https://europa.eu/eurobarometer/surveys/detail/2512(Acedido em 24-10-2021).
- Riad, A., Hocková, B., Kantorová, L., Slávik, R., Spurná, L., Stebel, A., Havriľak, M., & Klugar, M. (2021). Side Effects of mRNA-Based COVID-19 Vaccine: Nationwide Phase IV Study among Healthcare Workers in Slovakia. Pharmaceuticals (Basel, Switzerland), 14, 873.
