| Original Article, Biomed Biopharm Res., 2022; 19(1):153-167 doi: 10.19277/bbr.19.1.280; PDF version here [+] ; Portuguese html version [PT] |
Ionic liquids as tools to improve gel formulations containing sparingly soluble phenolic acids
Ana Júlio 1,2*, Nádia Remtula 3, Marisa Nicolai 1, Tânia Santos de Almeida 1,4*
1CBIOS-Universidade Lusófona’s Research Center for Biosciences & Health Technologies, Campo Grande 376, 1749–024 Lisboa, Portugal; 2Department of Biomedical Sciences, University of Alcalá, Ctra. Madrid-Barcelona Km. 33.600, Alcalá de Henares, 28871 Madrid, Spain; 3School of Sciences and Health Technologies, Lusófona University, Campo Grande 376, 1749-024 Lisboa, Portugal; 4Centro de Química Estrutural, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal
*corresponding authors:
Abstract
Hydroxycinnamic acids, namely caffeic and p-coumaric acids, have several pharmaceutical and cosmetic applications, but due to their low aqueous solubility, their applicability can be limited. Ionic liquids (ILs) have been shown to be a valuable tool to improve the solubility and assist in the incorporation of various phenolic compounds into delivery systems. Thus, this work aims to evaluate the impact of incorporating three choline-based ILs, namely (2-hydroxyethyl)-trimethylammonium-L-phenylalanine [Cho][Phe], (2-hydroxyethyl)-trimethylammonium-L-glutamate [Cho][Glu] and (2-hydroxyethyl)-trimethylammonium-glycinate [Cho][Gly], into an aqueous gel formulation containing the poorly soluble caffeic and p-coumaric acids. The results obtained confirm that the ILs not only increase the drug solubility, but also allow higher amounts of both studied drugs to be incorporated into the gels, without interfering with the stability of the aqueous gels.Moreover, the ILs altered the fluidity of the gels, both in the absence and presence of both phenolic acids, as they increase the viscosity of the formulations, contributing to higher flow resistance of the gel, which may be better accepted by the consumer.
Keywords: Ionic liquids; Hydroxycinnamic acids; Poorly soluble drugs; Gels; Increased Viscosity
Received: 31/01/2022; Accepted: 03/04/2022
Introduction
The phenolic compounds with one carboxylic acid group are one of the main classes of natural compounds extracted from plants, present in several seeds, fruits skin and vegetables leaves (1,2). These phenolic acids, also known as phenolcarboxylic acids, are subdivided in two groups: hydroxybenzoic (with seven carbon atoms) and hydroxycinnamic (with nine carbon atoms) acids (1–5).
The hydroxycinnamic acids are the most studied class of the phenolic acids (2) and the most common compounds within this class are ferulic, caffeic, p-coumaric and sinapinic acids (1,3,6). These acids have a wide distribution on natural resources, such as in fruits (apples, blueberries, oranges and pineapples), vegetables (lettuce, potatoes and spinach) and other herbs (marjoram, oregano and rosemary) (1,4–6).
Presently, due to consumers’ demands and environmental limitations, the pharmaceutical and cosmetic industries have searched for environmentally friendly resources as a source of drugs (1,6–8), such as the example of the hydroxycinnamic acids. This is one of the reasons for the growing number of works including these compounds. Several studies described the pharmaceutical and cosmetic activities of these molecules, such as antioxidant (5,9–11), anti-inflammatory (12), neuroprotective (13), anticancer (3,14,15), antilipidemic and antidiabetic (4,10). However, they present some limitations, due to their low solubility and bioavailability, which difficult their applicability (1,14,16–18).
In this context, ionic liquids (ILs) have been studied by our group as drug solubility promotors (14,17–20), namely with phenolic compounds (14,17,18). So, ILs can be used to improve drug loading of hydroxycinnamic acids into delivery systems. ILs are organic salts, that have melting points below 100 °C and some of them are liquids at room temperature (21–25).
In the pharmaceutical and cosmetic fields, they have been used with several goals, such as solvents and catalysts of active pharmaceutical excipients (26–29), as oil or water substitutes (26), as surfactants and viscosifiers in emulsions and microemulsions (14,17,30,31), as solubility enhancers (14,17–20,32) and integrated with nanoparticles (17,21,23,28,31). They act as a multifunctional tool since they may be tailored to achieve the most suitable properties, according with a desired applicability (14,24,28,33). And also, they present some properties that can be useful, for example high thermal and chemical stability, re-usability and nonflammability (22,23,28). However, it is also important to consider the toxicity of ILs. For instance, it has been shown, that choline-amino acid ILs are amongst the least toxic ILs (22) and may be more suited to be included in delivery systems. Nonetheless, several studies, performed in different cell lines, have shown that the toxicity of this class of ILs is concentration-dependent (14,18,22) and this should be considered when studying their applicability.
Concerning the hydroxycinnamic acids their topical applicability may be relevant due to some of their properties, already described, such as their antioxidant activity (2,4,7). Regarding this, emulsions and gels are quite suitable as topical delivery systems (7,19). In terms of emulsions, our group already showed the utility of using ILs containing amino acids to enhance the solubility of the hydroxycinnamic acids and they allowed a higher incorporation of these compounds in oil-in-water emulsions (14). Besides that, that study also proved that ILs can be useful to improve the stability and alter the viscosity of the developed topical delivery systems (14). Since emulsions are thermodynamically unstable formulations (7,19), in some cases, gels may be a suitable alternative, justifying their development in the presence of ILs.
Gels can be classified according to the nature of the solid phase, as colloidal, molecular, and polymeric (34,35). They may also be classified according to the liquid phase, as hydrogels (water), organogels (an organic solvent), polymer liquid gels, oleogels (oil) and ionic liquid gels (liquid phase constituted only by ILs). The combination of gels with ILs has led to improved functional materials that have been used in different areas, such as electronics, green chemistry, cosmetics and pharmaceutical,(34,35) showing the potential of this combination.
In the pharmaceutical and cosmetic fields, topical gels are widely used and these semisolid formulations, are formed by the dispersion of small or large molecules in an aqueous liquid vehicle that is jellified with the gelling agent addition (36). These vehicles may have aqueous, hydroalcoholic, alcohol or non-aqueous mean (36,37). Carbopol® is a gelling agent used as a thickener (38), emulsifier (39) and stabilizer (40) in topical formulations such as gels. In a preliminary study, we showed that ILs improved the incorporation of caffeine into Carbopol® gel formulations (19). Herein we assessed if ILs could also improve the incorporation of phenolic acids into this type of topical formulation. Nonetheless, although gels are more stable than emulsions, they may still have some stability problems, such as separation between the solids and the liquids components within the formulation and biological growth, especially gels containing water (19,41,42). Consequently, ILs may also be a valuable strategy to improve these formulations.
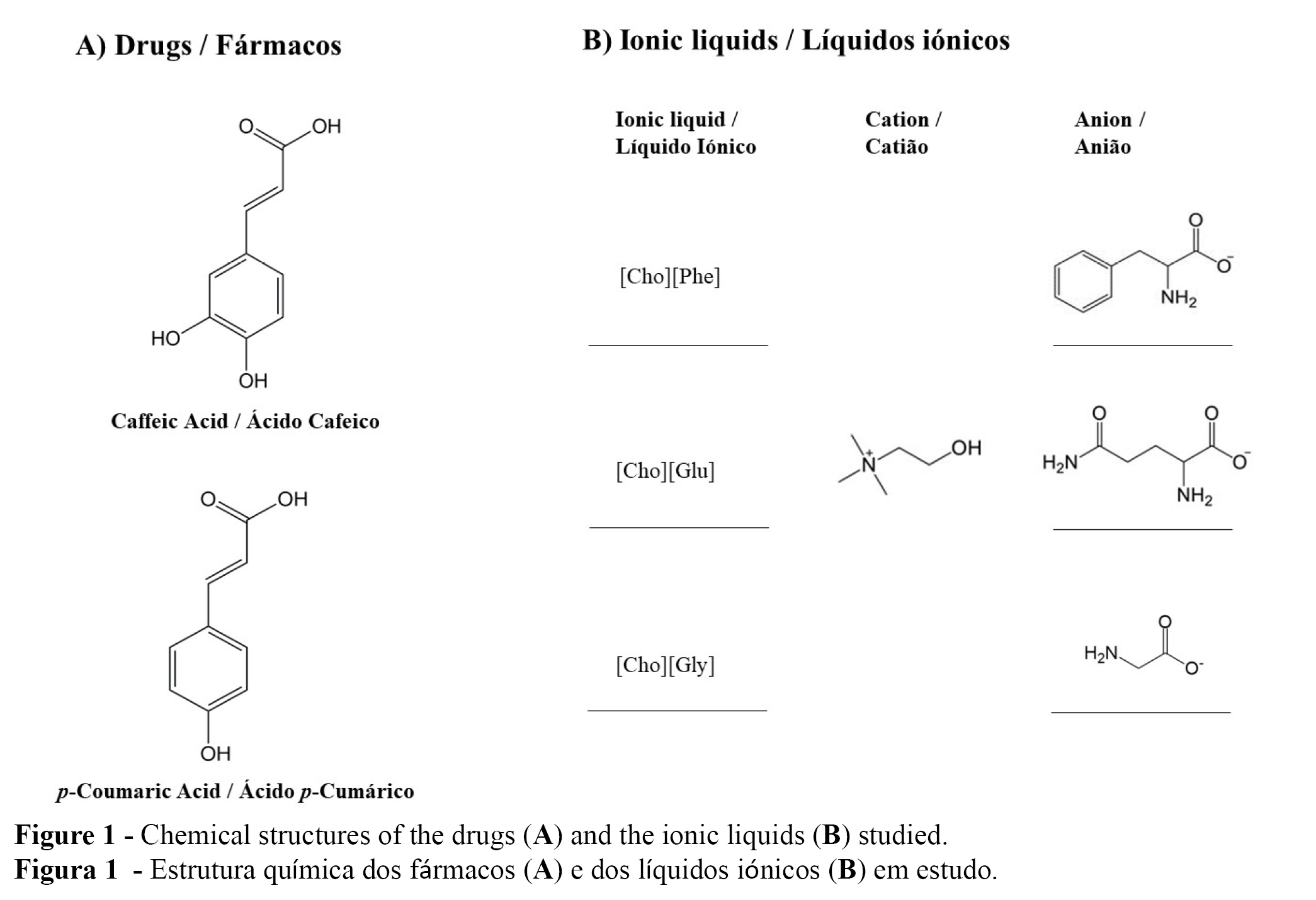
With all of this in mind, the goal of this work was to evaluate the performance of aqueous gels containing caffeic or p-coumaric acids (Figure 1A), in the presence and absence of three different choline-based ILs (Figure 1B). The impact of the ILs on the developed formulations and on the drug incorporation into those gels, was evaluated.
Materials and Methods
Materials and Reagents
Three choline based-ILs were used: (2-hydroxyethyl)-trimethylammonium-L-phenylalaninate [Cho][Phe], the (2-hydroxyethyl)-trimethylammonium-L-glutamate [Cho][Glu] and the (2-hydroxyethyl)-trimethylammonium-glycinate [Cho][Gly]. All ILs had been prepared within the context of other studies recently developed by our group (14,18).
The drugs chosen were two hydroxycinnamic acids, caffeic acid (CA) and p-coumaric acid (p-CA), (both Sigma-Aldrich, St. Louis, MO, USA). The gel formulation also included Carbopol® 940 and triethanolamine (both José Vaz Pereira, Benavente, Portugal), propylene glycol (Fragron, Barcelona, Spain) and the paraben concentrate, which was prepared according to the Farmacopeia Portuguesa VIII using methylparaben and propylparaben (both Sigma-Aldrich, St. Louis, MO, USA).
Synthesis of the Choline-based ILs
As mentioned above, the synthesis and all characterization of the studied ILs was performed within the scope of other studies conducted by our group (14,18). Briefly, each amino acid, 57.79 mmol, was solubilized in water and this solution was added to 57.76 mmol choline hydroxide, after evaporation. The resulting blend was stirred overnight. The solvent was then evaporated, followed by the addition of an acetonitrile:methanol (9:1). The obtained mixture was centrifuged, for 30 minutes at 1500 rpm, filtered, and then evaporated to remove the solvents. All the ILs were stored under moisture-free conditions and evaporated again before use.
Solubility Studies
Solubility studies were performed as previously described (14,18). Briefly, saturated solutions of each drug in water and water:IL mixtures (99.8:0.2% w/w) were prepared. The IL concentration used (0.2 %, v/v) allows the maintenance of cell viability of human keratinocytes (HaCaT cells), accordingly to previously published results (14,19). All solutions were prepared in triplicate.
These solutions then were mixed on a horizontal shaker (IKA VIBRAX VXR®, LTF Labortechnik GmbH & Co., Bodensee, Germany) for a period of 72 h at 25±2 °C. The solutions were then filtered. Finally, the samples were analyzed using a UV-visible spectrophotometer (Evolution® 300, Thermo Scientific, Hertfordshire, England), at the maximum absorption wavelength in water (286 nm for p-coumaric acid and 313 nm for caffeic acid).
Development of the aqueous gel formulation
The aqueous gel formulations were individually prepared (19) in the presence and absence of each studied IL ([Cho][Phe], [Cho][Glu] and [Cho][Gly]) and with and without each drug (caffeic or p-coumaric acids) (Table 1). All raw materials were previously weighed. The distilled water and the IL were placed in a beaker. The drug was then dissolved under stirring . After the drug was completely dissolved, the paraben concentrate, and propylene glycol were added. Finally, Carbopol® 940 was added under vigorous agitation and in shower mode until a homogeneous formulation was reached. After each formulation was prepared, the pH was adjusted with triethanolamine to pH 5 (19).

The organoleptic properties, pH and viscosity of all formulations were analyzed at time zero and after the preliminary stability studies. For the viscosity, all samples were held at 25 ºC, then homogenized using a glass rod for 2 minutes. Viscosity was then measured in a rheometer (DV3T from AMETEK®, Brookfield, Preston, United Kingdom) using a S19 probe. The pH was evaluated with a pH meter (Metrohm® 827, Herisau, Switzerland).
Preliminary stability studies of the produced formulations
All formulations (n=3) were submitted to a gradual heating test and a centrifugation test, according to the protocols described in the literature (14,17,41) to assess stability.
In the gradual heating test, 5.0 g of each gel was placed in a thermostatic bath, from an initial temperature of 40 ºC to a final temperature of 80 ºC, increasing the temperature 10 ºC every 30 minutes. A centrifugation test was also performed, in which 5.0 g of each formulation was heated at 45 ºC for 30 min and then centrifuged for 30 min at 7200×g.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5® (GraphPad Software, San Diego, MO, USA), using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test of differences in mean values of the results. Values were expressed as mean±standard deviation (SD). Differences between individual means were significant with * p<0.05 and *** p<0.001.
Results and Discussion
This work aimed to evaluate the impact of the incorporation of three different choline-based ILs, [Cho][Phe], [Cho][Glu] and [Cho][Gly], on aqueous gels in the absence or presence of poorly soluble caffeic or p-coumaric acids. All studies were performed incorporating an equal concentration of each IL to enable comparison. As a topical application is sought, the chosen concentration allows the maintenance of the cell viability on human keratinocytes (HaCaT cells), according to studies previously published by our group (14,19)
Solubility studies
To the best of our knowledge, the solubility of caffeic and p-coumaric acids in water:[Cho][Glu] (99.8:0.2 %w/w) is determined herein for the first time. This IL was selected by virtue of recent promising results of its incorporation into lipidic implants (25), allowing an improvement of the developed systems. Within this study we intended to explore its impact on another type of delivery system. The solubility of these phenolic acids in water and in the presence of water:[Cho][Phe] and water:[Cho][Gly] (99.8:0.2% w/w) has been previously studied (14). Nonetheless, all these solubilities were performed here for comparison and to ensure that the solubility of caffeic and p-coumaric acids in water:[Cho][Glu] (99.8:0.2 % w/w) was determined under the same experimental conditions.
The solubilities of the hydroxycinnamic acids in water at 25 ºC were determined to be 0.49±0.01 mg/mL for caffeic acid and 0.74±0.01 mg/mL for p-coumaric acid (Figure 2 and Table 2). For caffeic acid, this result is in agreement with a recently published study (0.45±0.04 mg/mL)(14) but slightly lower than the results obtained in a previous work (0.98±0.02 mg/mL) (16), although both studies described a solubility below 1 mg/mL. The results for the solubility of p-coumaric acid are in agreement with two studies presented in the literature (both with a solubility of 0.7 mg/mL) (14,43). For the solubility of both phenolic compounds in water: [Cho][Phe] (1.41±0.01 mg/mL for caffeic acid and 1.37±0.03 mg/mL for p-coumaric acid) and in water:[Cho][Gly] (1.61±0.04 mg/mL for caffeic acid and 1.56±0.01 mg/mL for p-coumaric acid), the current results are also in agreement with the previously published data (1.42±0.08 mg/mL with [Cho][Phe] and 1.39±0.07 mg/mL with [Cho][Gly] for caffeic acid and 1.39±0.02 mg/mL with [Cho][Phe] and 1.54±0.07 mg/mL with [Cho][Gly] for p-coumaric acid)(14) (Table 2).
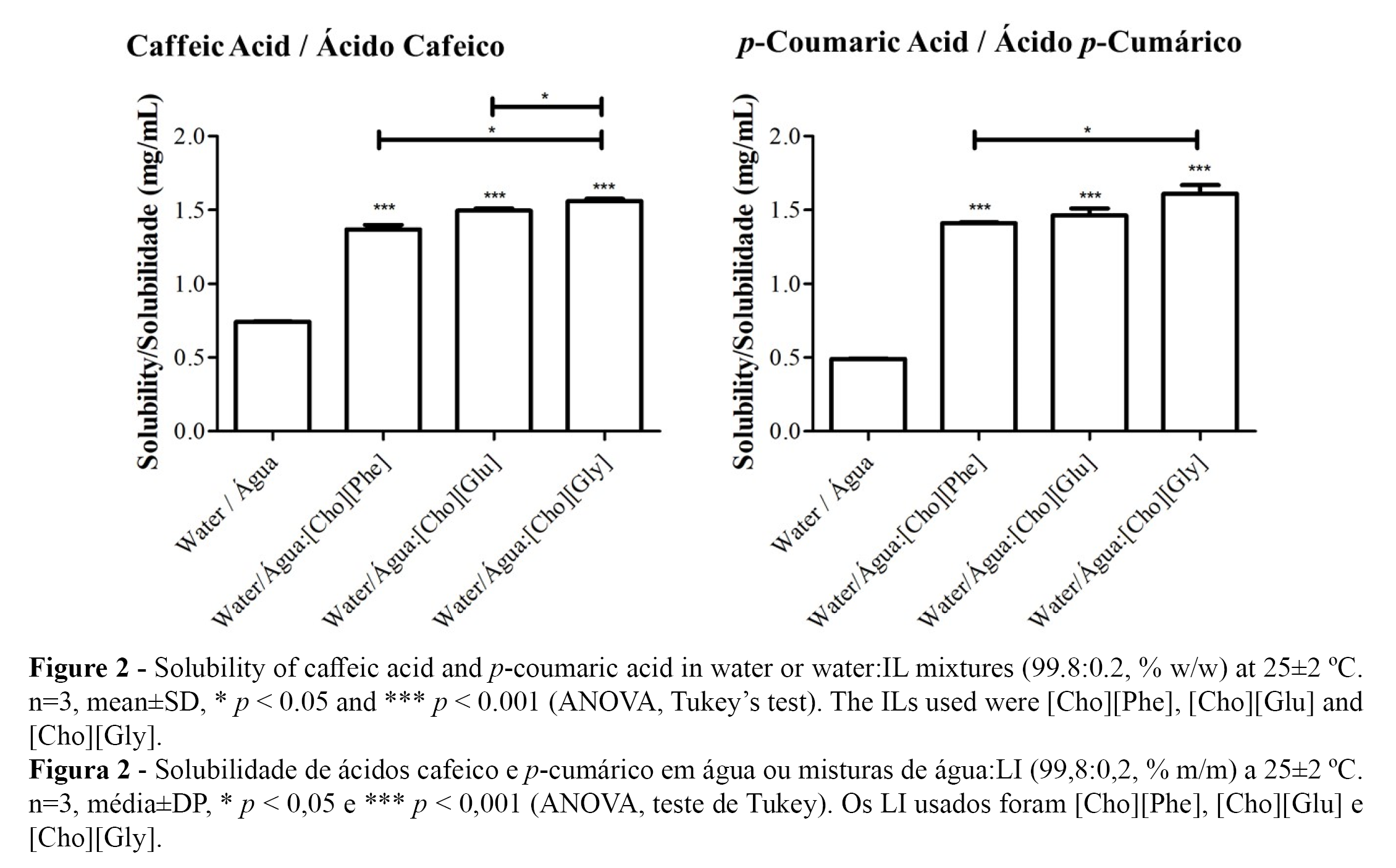

Concerning to the solubility of the phenolic compounds in water: [Cho][Glu] mixture (1.46±0.01 mg/mL for caffeic acid and 1.49±0.01 mg/mL for p-coumaric acid) proved to be quite similar to those obtained for the other choline-based ILs (Figure 2 and Table 2).
The solubility results, in the presence of the ILs, are all statistical different from the solubility results obtained for the phenolic acids in water alone (Figure 2 and Table 2).
This work, therefore, allowed us to conclude that [Cho][Glu] also improves the drug solubility of caffeic and p-coumaric acids and reinforces the fact that choline-based ILs may be useful as solubility promoters of sparingly soluble phenolic acids.
Following these results and considering that both drugs may have potential for a topical application (3,7,14), aqueous gel formulations were developed, in the presence and absence of each IL and/or each phenolic compound studied herein, to assess if whether these salts would have an impact on the developed formulations.
Development of gel formulations
For the preparation of the gel formulations, firstly the control gel was first prepared, containing no drug or IL. Subsequently, the drugs were incorporated into the gels individually, in the presence or absence of each IL. Each drug was incorporated in the formulations at the maximum (water) soluble concentration or in the corresponding water:IL mixture (99.8:0.2 % w/w), according to the results obtained in the solubility studies. Hence, several formulations were prepared, as described in Table 1, namely: a control gel, without IL or drug; gels containing each drug but without IL; gels incorporating each IL but without drug; and finally, the gels containing each IL and each drug.
Preliminary stability studies were carried out to assess if the prepared gels were stable and if the incorporation of ILs into the formulations would impact their stability. Thus, all the formulations were subjected to extreme conditions, through the gradual heating test and the centrifugation test. All gels were thermally and physically stable, indicating that the incorporation of ILs does not interfere with the stability of the aqueous gels. Moreover, the pH of all formulations was unaltered after the preliminary stability studies.
The organoleptic properties of the gels were observed macroscopically (Figure 3). All gel formulations are colourless and translucent (Figure 3).
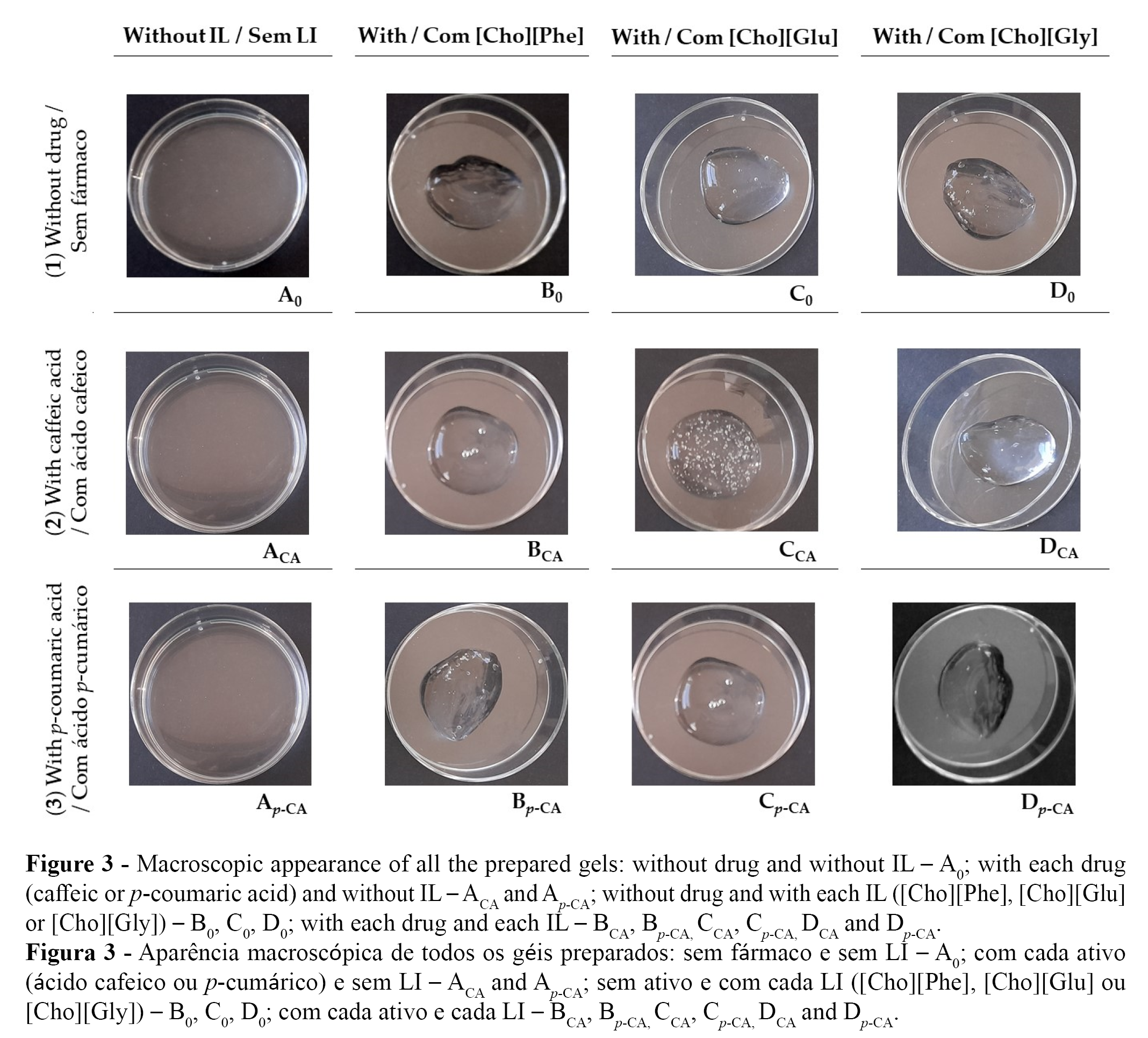
In terms of consistency, the formulations without IL (the control gel, A0, and the gels containing each drug but without IL, ACA and Ap-CA), presented a higher fluidity (Figure 3 A0, ACA and A p-CA).
On the other hand, the gels containing IL, either in the absence (B0, C0 and D0) or in the presence of each drug (BCA, CCA, DCA, Bp-CA, C p-CA and D p-CA) presented a less fluid and more pleasant appearance for a gel formulation (Figure 3 B0, C0 and D0). Thus, our results seem to indicate that the presence of the ILs may increase the viscosity of the prepared gels, either in the presence or in the absence of the studied drugs. This may be important, since a higher viscosity may contribute to a smaller flow velocity of the semi-solid or liquid formulations, due to the increase in the flow resistance (44,45). A less fluid gel may be more appealing to the consumer, since it is more practical to apply and may be more effective, due to longer skin contact time (36,37,44).
Consequently, the gel viscosities were then evaluated, and the results obtained confirmed the macroscopical observations (Figure 3), showing that the presence of the ILs led to a considerably increase in the viscosity of the formulations. Moreover, the control formulation - without drug and without IL - presented a viscosity similar to the formulations containing drug, but without IL (without statistical difference). On the other hand, the formulations containing ILs presented a considerably higher viscosity when compared to the formulations without IL (Table 3), indicating the viscosity of the developed formulations is affected by presence of the ILs rather than the presence of each active. Moreover, recently our group showed that ILs can stabilize oil-in-water emulsions by increasing their viscosity (14), which is in accordance with what was observed in this study with the gel formulations. Thus, this present study not only reveals that choline-based ILs increase the viscosity of gel formulations, but reinforces that these materials may be an innovative choice to alter the fluidity and stability properties of different topical formulations.

Finally, as the studied drugs were incorporated at the maximum concentration that they are soluble in water or in each water:IL mixture (99.8:0.2 %w/w), it is also important to note that the ILs not only contributed for a lower fluidity, but also improved the drug loading compared to the aqueous gels prepared in the absence of the ILs. This is due to the higher drug solubility in the presence of the ILs.
Conclusion
The results of the solubility studies carried out in this study showed that not only [Cho][Phe] and [Cho][Gly] may be used as solubility promotors of the studied phenolic compounds, but [Cho][Glu] also increases drug solubility by allowing a 2-fold drug solubility enhancement for p-coumaric acid and a 3-fold drug solubility enhancement for caffeic acid.
All the prepared gels were stable, showing that the ILs do not destabilize the formulations. Additionally, the developed gels in the presence of ILs presented a higher viscosity, a factor that may be considered in the formulation process and represent a new functionality of these salts in gel formulations.
Thus, in this study it was shown that choline-based ILs may be quite useful to enhance the drug loading of caffeic and p-coumaric acids into topical formulations, such as gels, and also shows that ILs may equally be used to alter the viscosity of topical systems, which may improve the consumer’s acceptance of the product.
Authors Contributions Statement
TSA, conceptualization and study design; AJ and NR, experimental implementation; AJ, NR and TSA, data analysis; AJ, MN and TSA, drafting, editing and reviewing; AJ, figures and graphics; TSA, supervision; AJ, MN and TSA, final writing.
Funding
This study was financially supported by Fundação para a Ciência e Tecnologia, through funding UIDB/0456/2020 and UIDP/04567/2020 (both general funding to CBIOS) as well as by funding from Universidade Lusófona/ILIND (Grant Programme FIPID 2019/2020).
Conflict of Interests
The authors declare there are no financial and/or personal relationship that may present a potential conflict of interest.
References
- Kumar, N., & Goel, N. (2019).Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology reports (Amsterdam, Netherlands), 24, e00370. https://doi.org/10.1016/j.btre.2019.e00370.
- Sova, M., & Saso, L. (2020). Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients, 12(8), 2190. https://doi.org/10.3390/nu12082190
- Caparica, R., Rolim Baby, A., Almeida, T.S., & Guilherme Costa, J. (2020).In vitrocytotoxicity assessment of ferulic, caffeic and p-coumaric acids on human renal cancer cells, Biomedical and Biopharmaceutical Research, 17(1), 63-74. https://www.alies.pt/BBR%20Editions/Vol-17-1-2020/bbr.17.1.225.pdf
- Coman, V., & Vodnar, D. C. (2020). Hydroxycinnamic acids and human health: recent advances. Journal of the science of food and agriculture, 100(2), 483–499. https://doi.org/10.1002/jsfa.10010
- Teixeira, J., Gaspar, A., Garrido, E. M., Garrido, J., & Borges, F. (2013).Hydroxycinnamic acid antioxidants: an electrochemical overview. BioMed research international, 2013, 251754. https://doi.org/10.1155/2013/2517546.
- Martinez, K. B., Mackert, J. D. and McIntosh, M. K. (2017) Polyphenols and intestinal health In R. R. Watson (Ed.), Nutrition and Functional Foods for Healthy Aging (Chapter 18., pp. 191-210) Academic Press.
- Taofiq, O., González-Paramás, A. M., Barreiro, M. F., & Ferreira, I. C. (2017).Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules (Basel, Switzerland), 22(2), 281. https://doi.org/10.3390/molecules22020281.
- Nicolai, M., Mota, J., Fernandes, A. S., Pereira, F., Pereira, P., P Reis, C., Robles Velasco, M. V., Baby, A. R., Rosado, C., & Rijo, P. (2020).Assessment of the Potential Skin Application of Plectranthus eckloniiBenth. Pharmaceuticals (Basel, Switzerland), 13(6), 120. https://doi.org/10.3390/ph13060120
- Peres, D. D., Ariede, M. B., Candido, T. M., de Almeida, T. S., Lourenço, F. R., Consiglieri, V. O., Kaneko, T. M., Velasco, M. V., & Baby, A. R. (2017).Quality by design (QbD), Process Analytical Technology (PAT), and design of experiment applied to the development of multifunctional sunscreens. Drug development and industrial pharmacy, 43(2), 246–256. https://doi.org/10.1080/03639045.2016.1236809
- Alam, M. A., Subhan, N., Hossain, H., Hossain, M., Reza, H. M., Rahman, M. M., & Ullah, M. O. (2016). Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutrition & metabolism, 13, 27. https://doi.org/10.1186/s12986-016-0080-3
- Kiokias, S., Proestos, C., & Oreopoulou, V. (2020). Phenolic Acids of Plant Origin-A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods (Basel, Switzerland), 9(4), 534. https://doi.org/10.3390/foods9040534.
- Kim, E. O., Min, K. J., Kwon, T. K., Um, B. H., Moreau, R. A., & Choi, S. W. (2012). Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 50(5), 1309–1316. https://doi.org/10.1016/j.fct.2012.02.011
- Zhang, X., He, X., Chen, Q., Lu, J., Rapposelli, S., & Pi, R. (2018). A review on the hybrids of hydroxycinnamic acid as multi-target-directed ligands against Alzheimer's disease. Bioorganic & medicinal chemistry, 26(3), 543–550. https://doi.org/10.1016/j.bmc.2017.12.042
- Caparica, R., Júlio, A., Fernandes, F., Araújo, M., Costa, J. G., & Santos de Almeida, T. (2021).Upgrading the Topical Delivery of Poorly Soluble Drugs Using Ionic Liquids as a Versatile Tool. International journal of molecular sciences, 22(9), 4338. https://doi.org/10.3390/ijms22094338
- Damasceno, S. S., Dantas, B. B., Ribeiro-Filho, J., Antônio M Araújo, D., & Galberto M da Costa, J. (2017).Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy.A Review. Current pharmaceutical design, 23(20), 3015–3023. https://doi.org/10.2174/1381612822666161208145508
- Mota, F., Queimada, A.J., Pinho, S.P., & Macedo, E.A. (2008).Aqueous Solubility of Some Natural Phenolic Compounds. Industrial & Engineering Chemistry Research, 47, 5182-5189.
- Caparica, R., Júlio, A., Baby, A. R., Araújo, M., Fernandes, A. S., Costa, J. G., & Santos de Almeida, T. (2018).Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics, 10(4), 288. https://doi.org/10.3390/pharmaceutics10040288
- Caparica, R., Júlio, A., Araújo, M., Baby, A. R., Fonte, P., Costa, J. G., & Santos de Almeida, T. (2020).Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells.Biomolecules, 10(2), 233. https://doi.org/10.3390/biom10020233
- Santos de Almeida, T., Júlio, A., Saraiva, N., Fernandes, A. S., Araújo, M., Baby, A. R., Rosado, C., & Mota, J. P. (2017).Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: cytotoxicity, solubility, and skin permeation studies.Drug development and industrial pharmacy, 43(11), 1858–1865. https://doi.org/10.1080/03639045.2017.1349788
- Júlio, A., Antunes, C.D., Mineiro, R., Raposo, M., Caparica, R., M. Araújo, M.E., Rosado, C., Fonte, P., & Santos de Almeida, T. (2018).Influence of two choline-based ionic liquids on the solubility of caffeine. Biomedical and Biopharmaceutical Research, 15(1):96–102. https://www.alies.pt/BBR%20Editions/Vol-15-1-2018/art9.pdf
- Júlio, A., Caparica, R., Costa Lima, S. A., Fernandes, A. S., Rosado, C., Prazeres, D., Reis, S., Santos de Almeida, T., & Fonte, P. (2019).Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials (Basel, Switzerland), 9(8), 1148. https://doi.org/10.3390/nano9081148.
- Gouveia, W., Jorge, T. F., Martins, S., Meireles, M., Carolino, M., Cruz, C., Almeida, T. V., & Araújo, M. E. (2014).Toxicity of ionic liquids prepared from biomaterials. Chemosphere, 104,51–56. https://doi.org/10.1016/j.chemosphere.2013.10.055
- Santos de Almeida, T., Júlio, A., Mota, J. P., Rijo, P., & Reis, C. P. (2017).An emerging integration between ionic liquids and nanotechnology: general uses and future prospects in drug delivery. Therapeutic delivery, 8(6), 461–473. https://doi.org/10.4155/tde-2017-0002
- Pedro S.N., Freire, C.S.R., Silvestre, A.J.D., Freire, M.G. (2021) Ionic Liquids in Drug Delivery. Encyclopedia, 1(2):324-339. https://doi.org/10.3390/encyclopedia1020027
- Júlio, A., Sultane, A., Viana, A. S., Mota, J. P., & Santos de Almeida, T. (2021).Biobased Ionic Liquids as Multitalented Materials in Lipidic Drug Implants. Pharmaceutics, 13(8), 1163. https://doi.org/10.3390/pharmaceutics13081163
- Silva, W., Zanatta, M., Ferreira, A. S., Corvo, M. C., & Cabrita, E. J. (2020).Revisiting Ionic Liquid Structure-Property Relationship: A Critical Analysis. International journal of molecular sciences, 21(20), 7745. https://doi.org/10.3390/ijms21207745
- Ferraz, R., Silva, D., Dias, A. R., Dias, V., Santos, M. M., Pinheiro, L., Prudêncio, C., Noronha, J. P., Petrovski, Ž., & Branco, L. C. (2020).Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin G and Amoxicillin hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics, 12(3), 221. https://doi.org/10.3390/pharmaceutics12030221.
- Pedro, S. N., R Freire, C. S., Silvestre, A., & Freire, M. G. (2020).The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. International journal of molecular sciences, 21(21), 8298. https://doi.org/10.3390/ijms21218298.
- Siopa, F., Frade, R.F., Diniz, A.M., Andrade, J.M., Nicolai, M., Meirinhos, A.R., Lucas, S.D., Marcelo, F., Afonso, C.A., & Rijo, P. (2018).Acetylcholinesterase Choline-Based Ionic Liquid Inhibitors: In Vitro and in Silico Molecular Docking Studies. ACS Omega, 3(12):17145–54.
- Ali, M.K., Moshikur, R.M., Wakabayashi, R., Moniruzzaman, M., Kamiya, N., & Goto, M. (2020). Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustainable Chemistry & Engineering, 8, 6263-6272.
- Júlio, A., Caparica, R., Costa Lima, S. A., Fernandes, A. S., Rosado, C., Prazeres, D., Reis, S., Santos de Almeida, T., & Fonte, P. (2019).Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials (Basel, Switzerland), 9(8), 1148. https://doi.org/10.3390/nano9081148
- Almeida, T.S., Júlio, A., Caparica, R., Rosado, C., Fernandes, A.S., Saraiva, N., Ribeiro, M., Araujo, M., Baby, A.R., Costa, J., & Mota, J.P. (2015).Ionic liquids as solubility/permeation enhancers for topical formulations: Skin permeation and cytotoxicity characterization. Toxicology Letters, 238..
- Moshikur, R.M., Chowdhury, M.R., Moniruzzaman, M., & Goto, M. (2020). Biocompatible ionic liquids and their applications in pharmaceutics. Green Chemistry, 22, 8116-8139.
- Flory, P. J. (1974) Introductory lecture. Faraday Discussions of the Chemical Society, 57(1):7–18. https://doi.org/10.1039/DC9745700007
- Marr, P.C., & Marr, A.C. (2016). Ionic liquid gel materials: applications in green and sustainable chemistry. Green Chemistry, 18, 105-128.
- Sharadha, M., GowdaD, V., VishalGupta, N., & AkhilaA, R. (2020). An overview on topical drug delivery system – Updated review. International Journal of Research in Pharmaceutical Sciences, 11, 368-385.
- Singh Malik, D., Mital, N., & Kaur, G. (2016). Topical drug delivery systems: a patent review. Expert Opinion on Therapeutic Patents, 26, 213 - 228..
- Das, B., Nayak, A.K., & Nanda, U. (2013).Topical gels of lidocaine HCl using cashew gum and Carbopol 940: preparation and in vitro skin permeation. International journal of biological macromolecules, 62,514-7 .
- Lee, J. A., & Nobles, W. L. (1959). Pharmaceutical applications of the sodium salt of carbopol 934. Journal of the American Pharmaceutical Association. American Pharmaceutical Association, 48(2), 92–94. https://doi.org/10.1002/jps.3030480205.
- Chen, J., Zhou, R., Li, L., Li, B., Zhang, X., & Su, J. (2013). Mechanical, rheological and release behaviors of a poloxamer 407/ poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules (Basel, Switzerland), 18(10), 12415–12425. https://doi.org/10.3390/molecules181012415.
- ANVISA - National Health Surveillance Agency. Cosmetic Products Stability Guide. Vol. 1. Brasilia, Brazil: ANVISA Publising House; 2004.
- Dervaux, J., & Amar, M. (2012). Mechanical Instabilities of Gels. Annual Review of Condensed Matter Physics, 3(1), 311-332..
- Fontecha-Cámara, M. A., Álvarez, M. A., López-Ramón, V., & Moreno-Castilla, C. (2015). Fenton oxidation of gallic and p-coumaric acids in water assisted by an activated carbon cloth. Water science and technology : a journal of the International Association on Water Pollution Research, 71(5), 789–794. https://doi.org/10.2166/wst.2015.034.
- Calixto, L. S., Infante, V., & Maia Campos, P. (2018).Design and Characterization of Topical Formulations: Correlations Between Instrumental and Sensorial Measurements. AAPS PharmSciTech, 19(4), 1512–1519. https://doi.org/10.1208/s12249-018-0960-0.
- Correa, N.M., Junior, F.B., Ignácio, R.F., & Leonardi, G.R. (2005).Avaliação do comportamento reológico de diferentes géis hidrofílicos. Revista Brasileira De Ciencias Farmaceuticas, 41,73-78.
