| Original Article, Biomed Biopharm Res., 2022; 19(2):379-496 doi: 10.19277/bbr.19.2.297; PDF version here [+] ; Portuguese html version [PT] |
Oral intake of Bioactive Collagen Peptides in the improvement of skin and hair: clinical studies by instrumental measurements
Patricia M. B. G. Maia Campos*, Maísa Oliveira de Melo, Marina Mendes Fossa Shirata, Marcella Gabarra Leite
Faculdade de Ciências Farmacêuticas de Ribeirão Preto da Universidade de São Paulo. Av. do Café S/N – CEP: 14040-903, Monte Alegre, Ribeirão Preto, Brasil
* corresponding author:
Abstract
The effect of collagen peptides on various aspects of skin and hair physiology is known but needs further studies. Thus, the objective of this study was to evaluate the clinical changes in skin and hair after 90 days of treatment with oral supplementation of 5 g/day of collagen peptides. For this, 60 healthy female participants were enrolled, aged 45 to 60 years old, with the primary objective of evaluating the effect of the ingestion of a bioactive collagen peptides-based supplementation in the cutaneous microrelief, reduction of wrinkles, thickness, and echogenicity of the dermis, as well as in the mechanical properties of the hair using biophysical and skin imaging techniques. The present study showed important benefits in relevant skin visual parameters, dermis density, and hair strength with the obtained data. In addition, considering that the aging process affects the hair's mechanical resistance due to hair fiber thinner, the proposed treatment was effective for aged hair. In conclusion, the collagen peptides oral supplementation is essential not only for improving skin conditions but also for hair care once it significantly increases the mechanical hair resistance evaluated by objective measurements.
Keywords: Collagen peptides, skin aging, skin imaging techniques, hair mechanical properties, clinical study
Received: 12/10/2022; Accepted: 07/11/2022
Introduction
To be considered a healthy individual, a few different parameters are analyzed, such as physical exercises, sleep habits, presence of addictions, and others, but one of the best-known facts regarding this subject is that overall health is closely associated with nutritional habits. A balanced diet, comprising all necessary macro- and micronutrients, is vital for preventing a myriad of diseases. This is especially true for health problems that are associated with aging, such as cardiovascular disease. In this context, the skin is the first and most apparent indicator to reflect an individual’s health. Smooth, clean, and young-looking skin is perceived as a desirable attribute, so treating the signs of skin aging is an important concern for many and can be a key motivation for a healthy diet. Many micronutrients, such as Vitamin C, E, biotin, and zinc, have been associated with direct benefits for skin physiology and are used as ingredients for dietary supplements with the aim of reducing signs of skin aging (1,2).
During the aging process, the skin becomes less hydrated, losing its elasticity and firmness, along with increased pigmentation, pore size, and other structural changes in the dermis and epidermis. Furthermore, the collagen network in the dermis that provides strength and firmness to the skin becomes thinner and more fragmented (3). An increase in matrix metalloproteinase (MMP) expression accounts for accelerated collagen degradation (1,3). With a less dense and more fragmented network of collagen fibers, the embedded fibroblasts receive fewer mechanical stimuli, a key trigger for their metabolic activity. In addition, the synthesis of new extracellular matrix components from fibroblasts inherently slows down with age. Both processes prevent the degraded matrix from being adequately replaced (4). The elastic fibers of the papillary dermis lose integrity during aging and reach less far into the dermal-epidermal junction. This overall loss in elasticity, hydration, and strength leads to visual characteristics such as sagging and wrinkle formation (1,3).
Targeted enzymatic hydrolysis of collagen produces a natural combination of peptides with a defined mean molecular weight that carries bioactivity in supporting different biological processes that contribute to a healthy aging process. Collagen is the most abundant protein in the body, present in the extracellular matrix of all connective tissues. Thus, evidence at the preclinical and clinical levels is building up for hydrolyzed collagens to support the maintenance of the musculoskeletal system, including bones, ligaments, tendons, and joints. Collagen has a unique amino acid composition with a specifically high abundance of glycine, proline, and hydroxyproline. Upon ingestion, hydrolyzed collagen is digested into free amino acids and di- and tripeptides resistant to further hydrolysis by intracellular or serum peptidases (5). These peptides are transported across the intestinal barrier by a designated transporter, PEPT-1 (6). Hydroxyproline-containing peptides derived from hydrolyzed collagen ingestion have been identified in human serum with a peak of absorption one hour after the ingestion (7). A topic still under discussion is whether peptides longer than two or three amino acids can pass the intestinal barrier. Studies with radioactively labeled collagen-derived peptides demonstrated the efficient transport and uptake into target tissues, such as bone, cartilage, muscle, and skin (8). Astonishingly, these peptides could be detected in the skin up to 14 days after administration (9).
Hydrolyzed collagen can be extracted from different sources and tissues. Bovine collagen can be extracted from the bovine Achilles tendon using different enzymes such as alcalde, pepsin, trypsin, and collagenase produced by Penicillium aurantiogriseum. It is known for having antihypertensive, antioxidant, and antimicrobial activity. When obtained from bovine lung, it has antioxidant and anti-inflammatory activity. Finally, hydrolyzed collagen from the nuchal ligament of bovine can be used as a promising precursor of the angiotensin-I-converting enzyme (ACE)-inhibitory peptides (10).
Collagen obtained from porcine skin is also produced and shows antioxidant, anti-aging, and skin permeation properties.
However, these two collagen sources have some limitations due to health problems such as swine flu and bovine spongiform encephalopathy. Furthermore, religious issues should also be considered. Thus, alternative sources from marine sources have been developed, especially from fish and other invertebrates such as jellyfish or sponges. They present nutraceutical effects due to their functional bioactive properties. In some cases, antioxidant and antimicrobial activity are also present (10).
The effect of hydrolyzed collagen on various aspects of skin physiology has been investigated in preclinical studies. Oral supplementation with hydrolyzed collagen inhibited the loss and the fragmentation of collagen by aging in rats, in part by inducing the expression of type I and type III collagen, as well as inhibiting MMP expression and activity (1,3). In pigs, hydrolyzed collagen intake increased collagen fibrils' density and diameter (11). Similar effects on the stimulation of collagen synthesis were observed in cultured fibroblasts isolated from UV-exposed body areas (12). UV-induced damage to the skin was hampered in mice by inhibiting inflammatory pathways (13). Further, at a cellular level, hydrolyzed collagen has been shown to promote skin fibroblasts' growth and induce fibroblast migration (7).
Several clinical studies using various designs have shown that hydrolyzed collagens effectively improve skin aging parameters. In this line, oral supplementation with hydrolyzed collagen has been reported to improve skin hydration (14-17), elasticity (14,17,18), and dermal collagen density (1,16). In addition, a randomized, controlled trial showed that hydrolyzed collagen supplementation reduced the fragmentation of the collagen network in the dermis, a key denominator of aging, indicating that the collagen network's quality was improved (17,19).
Previous studies from our research group have recently investigated the efficacy of a formulation containing 10 g of bovine collagen peptides, Vitamins A, C, E, and zinc in a Brazilian population (1). Daily supplementation over a period of 90 days demonstrated the improvement of dermal collagen density, skin elasticity, and a reduction of wrinkles and pores.
Furthermore, considering that about 65 to 95% of the hair composition is of proteins, oral supplementation with Bioactive Collagen peptides can also be used to provide benefits to hair fiber. With the aging process, there is a reduction in the quality of the collagen fibers, which is noticed mainly in the skin appearance. The aging process also presents an effect on the hair which can be observed as a reduction in hair diameter, which can promote the weakening of the hair fiber (20,21). This is reflected primarily as a decrease of the hair break force with the aging process and a decrease in shiny hair appearance (21). Thus, the use of collagen peptides can be used as a support to improve the quality of the skin and its appendices.
Oral supplements have been a trend in the cosmetic field to repair skin and hair appearance and structure. Thus, several studies have reported that using collagen supplementation can improve skin functions and delay aging (22). Collagen supplementation has also been reported to positively influence the nails (23). At the same time, there are no reports in the scientific literature regarding the benefits of using collagen to the hair fiber, although this product has already been used for this purpose.
The current scope of clinical research has been predominantly performed in Caucasian (European) (16,18) and Asian (Japanese) (14-17) populations. However, skin aging differs across skin types, and in addition to ethnicity, regional impacts such as the climate can be key factors that determine how the skin ages (24). The alterations in the hair fiber also differ according to ethnicity and hair type, e.g., it has been reported that curly hair presents more fragility to hair breakage than other hair types (21). Thus, the clinical investigation of the benefits of Bioactive Collagen peptides supplementation in the Brazilian population, which presents excellent diversity in its ethnicity, is a significant contribution to the scientific field.
In addition, all previously investigated products were of fish or porcine but not of bovine origin. The bovine hide is one of the most important sources of raw material to produce collagen in Brazil due to the country's huge cattle herd, making this type of product more available for Brazilian and South American women than other sources. This way, the present study has an innovative proposal, investigating the efficacy of bovine collagen peptides without the addition of different components and with a lower dose, 5 g/day, not previously studied from the bovine source, and focusing primarily on dose-dependent dermal collagen density results, wrinkles, skin roughness, and hair strength. This final parameter has not been previously evaluated in a study using collagen peptides.
In this context, the objective of this study was to evaluate the clinical changes in skin and hair after 90 days of treatment with oral supplementation of bioactive collagen peptides. In summary, this study has an innovative proposal since it shows the clinical efficacy of collagen peptides oral supplementation in improving skin and hair in a non-invasive way.
Materials and Methods
After the approval by the Ethics Committee in Clinical Research of the School of Pharmaceutical Sciences of Ribeirão Preto/SP (CEP / FCFRP nº439 - CAAE nº 65109317.2.0000.5403), 60 healthy female participants were enrolled, aged 45 to 60 years old (mean age 53.4 ± 4.2 years old), level 3 of the Glogau Scale and Fitzpatrick phototype scale II-III. Most study participants had a small to medium percentage of white hair but used coloring procedures. The main objective was to evaluate the effect of the ingestion of a collagen-based supplementation in the cutaneous microrelief, reduction of wrinkles and thickness, and echogenicity of the dermis, as well as in the mechanical properties of the hair using biophysical and skin imaging techniques.
The study products were maltodextrin for the placebo group and bovine collagen peptides (Peptan® B, Rousselot, Amparo, Brazil) for the treatment group. The study period was 90 days, with three evaluations; one was before (baseline-T0) and the other after 45 and 90 days of treatment. Participants signed the Informed Consent Term before accepting participation in the study after a meeting to elucidate and clarify possible questions of the interested participants. All participants also received the same sunscreen (SPF 60) to use and avoid changes caused by using different products. All participants were also requested to stop using other cosmetic products for 15 days before the start of the study and during the whole treatment.
After assessing the inclusion and exclusion criteria, accepting to participate in the study, and signing the informed consent, the participants were divided into two groups: the placebo group, who ingested 5 g / day of a maltodextrin-based food, and the treatment group, who consumed 5 g / day of the oral supplementation under study. A template was standardized by the research group to ensure that the same area was analyzed after 90 days of treatment.
Hair samples of the participants of the study were collected before the beginning of the treatment (T0) and at the end (T90) to perform the characterization test. The samples were collected as close as possible to the root to evaluate the region of growth of the hair, where the alterations would be more evident. The control sample (without any treatment, T90) was kept to evaluate the hair compared to those that received the treatment after 90 days.
Biophysical and skin imaging techniques
Skin microrelief
The SELS (Surface Evaluation of the Living Skin) method is based on a graphic created of the living skin picture under special illumination. The microrelief parameters were evaluated using Visioscan® VC98 and software SELS 2000 from Courage & Khazaka Electronic Gmbh (Cologne, Germany). The measurement area was 6 × 8 mm2, and the skin image was taken by a built-in CCD camera. The electronic processing and evaluation of this image were conducted according to four clinical parameters: a) Skin Smoothness (Sesm ) - calculated from the average width and depth of wrinkles, b) Skin roughness (Ser) - the skin roughness parameter, calculated by the gray levels above the threshold in comparison with the entire image (reflects the ‘asperity’ of the skin.), c) Wrinkles (Sew) – calculated from the proportion of horizontal and vertical wrinkles and d) Wrinkle depth (Rt) (24). All measurements were made in the malar region of the face.
Measurement of the skin by high-resolution photography
The Visioface® digital photography imaging system (Courage and Khazaka, Germany) was utilized to evaluate facial skin. It consists of a cabin attached to a high-resolution digital camera (10 megapixels) and 200 white LED lights. This device is connected to research software that evaluates visible pores and wrinkles (1). The wrinkle analysis was evaluated on a 5 point scale in both the nasolabial and periorbital regions.
Measurement of dermis echogenicity
To evaluate the dermis echogenicity, 20 MHz ultra-sound equipment (Dermascan® C, Cortex Technology, Aalborg, Denmark) was chosen. It is based on the principle that the ultrasonic wave (speed of 1,580 m/s) is partially reflected by the skin structure, creating echoes of different amplitudes. To calculate the echogenicity, the number of pixels with low echogenicity is measured utilizing the image analysis software and related to the total number of pixels (1). All measurements were also taken in the malar region of the face.
Hair Characterization Studies
Tensile Test
The hair was evaluated in terms of break force and was performed using the equipment TA.XT Plus Texture Analyzer® (Stable Microsystems, Surrey UK). The analysis was performed in a room at a 20-22°C and 50-60% relative humidity (RH). The wire diameters were measured with a dynamometer, and 20 fibers of similar diameter at least 10 cm long were selected. The 20 wires were submitted individually to the rupture test in the Texturometer equipment at 55 mm distance, 10N load, and a constant rate of 300 mm/min (25).
Statistical analysis
Two-way ANOVA and Bonferroni post-test were used to evaluate the results obtained in this study. Statistical differences between placebo and collagen peptides groups were analyzed by the paired student’s T-tests, which as also used for basal and T90 measurements of each parameter (GraphPad Software Inc., La Jolla, CA, USA). Differences were accepted as statistically significant at p < 0.05.
Results
Biophysical and skin imaging techniques
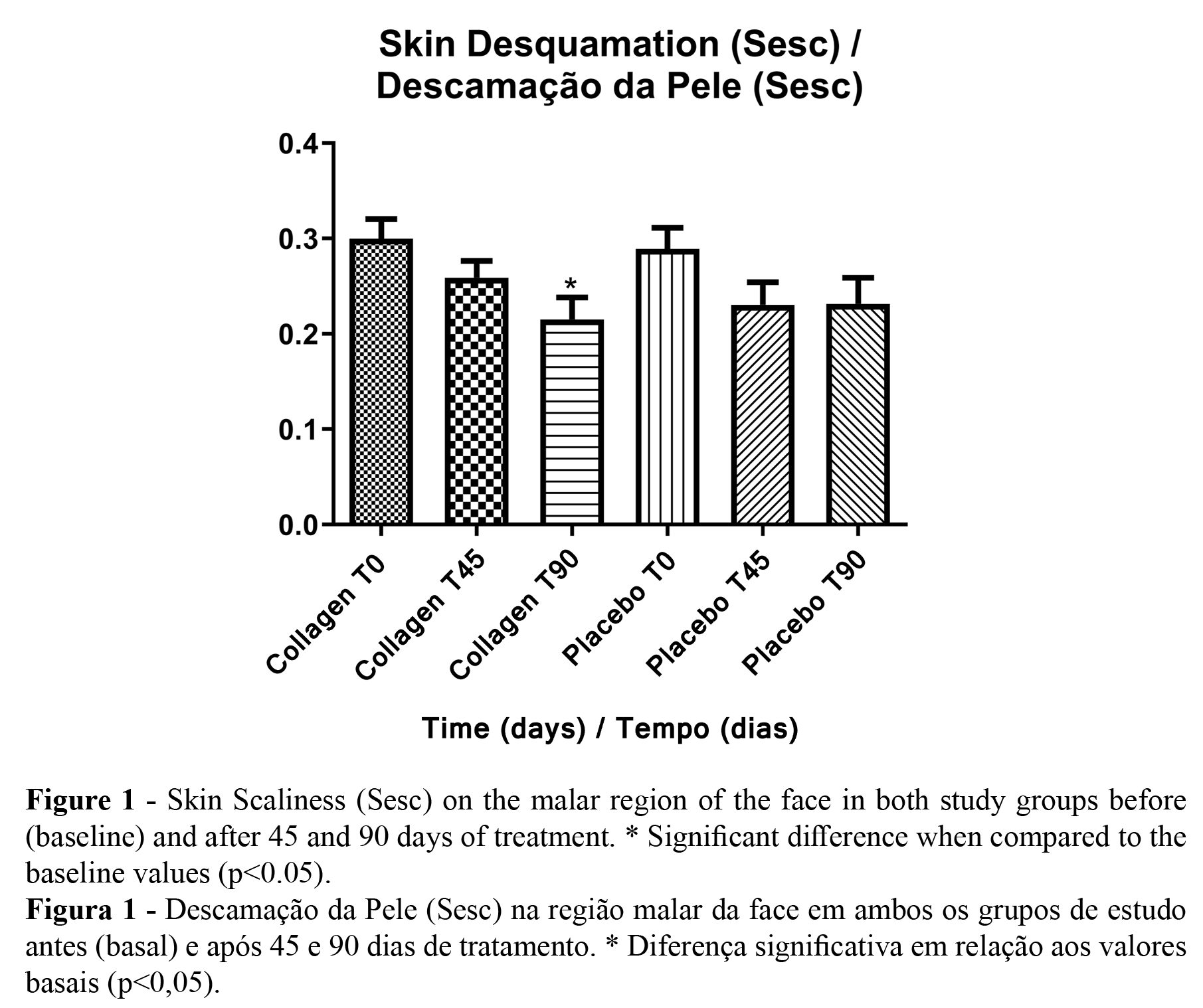
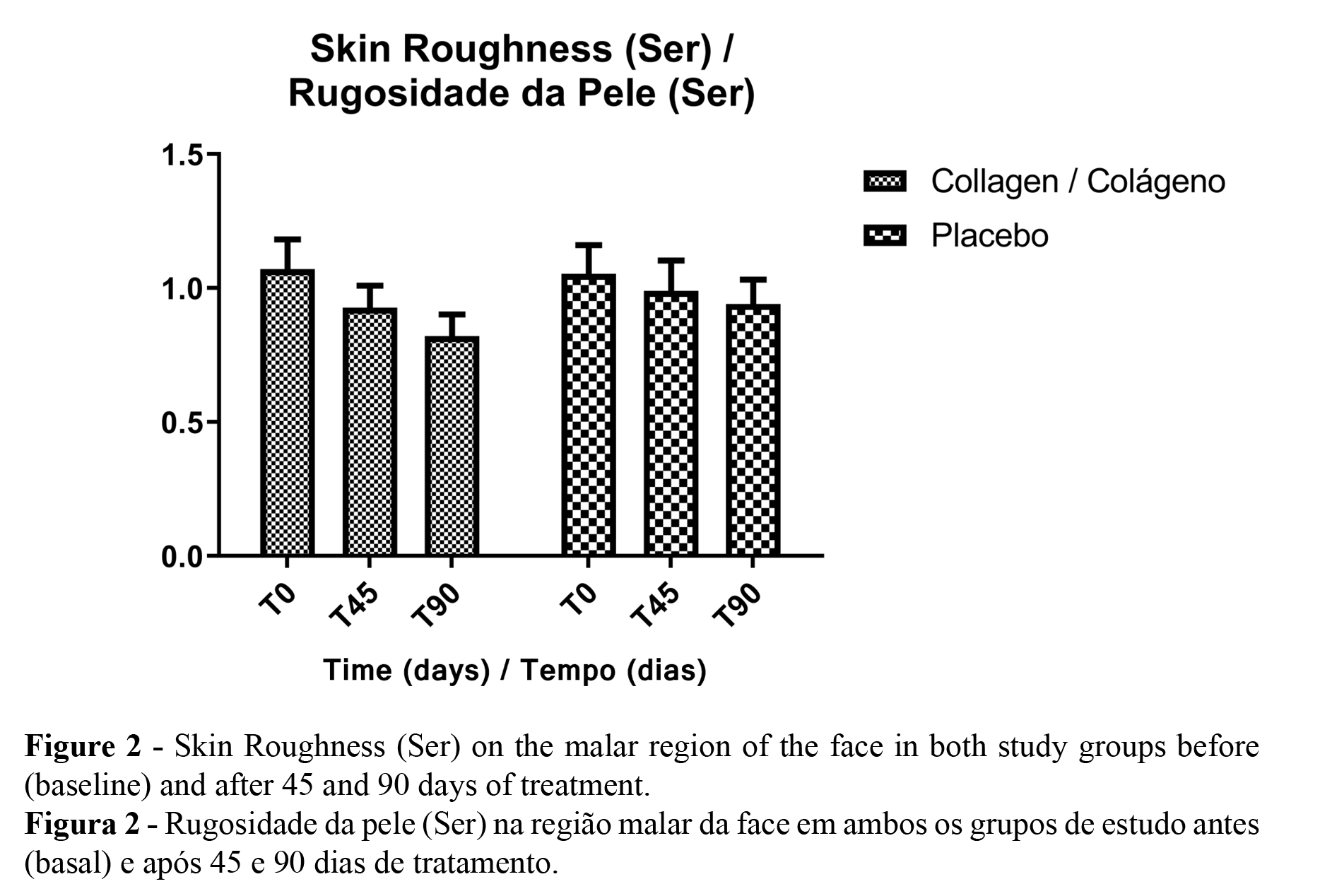
According to the microrelief analysis, it was possible to observe a difference in the parameters related to skin scaliness (Sesc), roughness (Ser), and smoothness (Sesm) only in the collagen peptides treatment group after 90 days of treatment. The only significant parameter was the Sesc. In the placebo group, no significant difference was noted (Figures 1 and 2). Figures 3 and 4 represent participants in each study group.


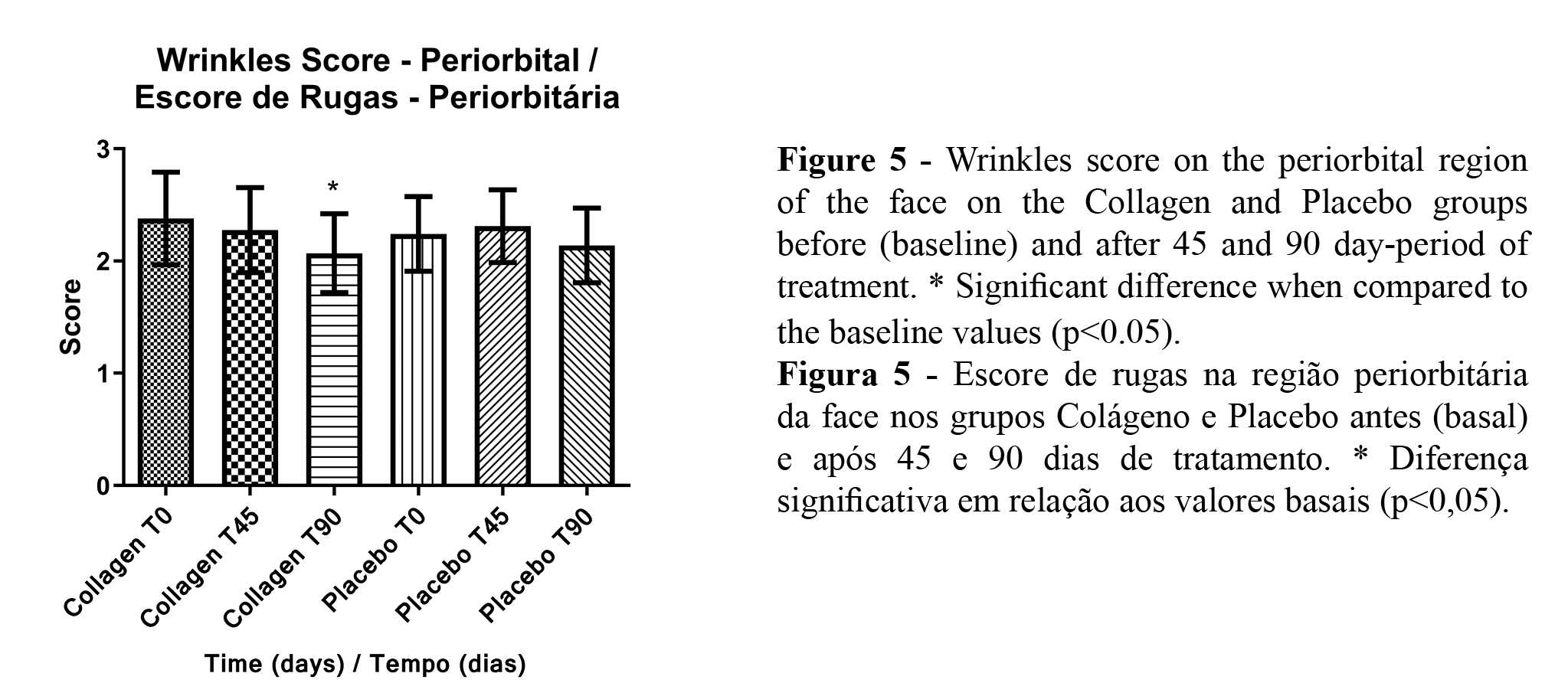
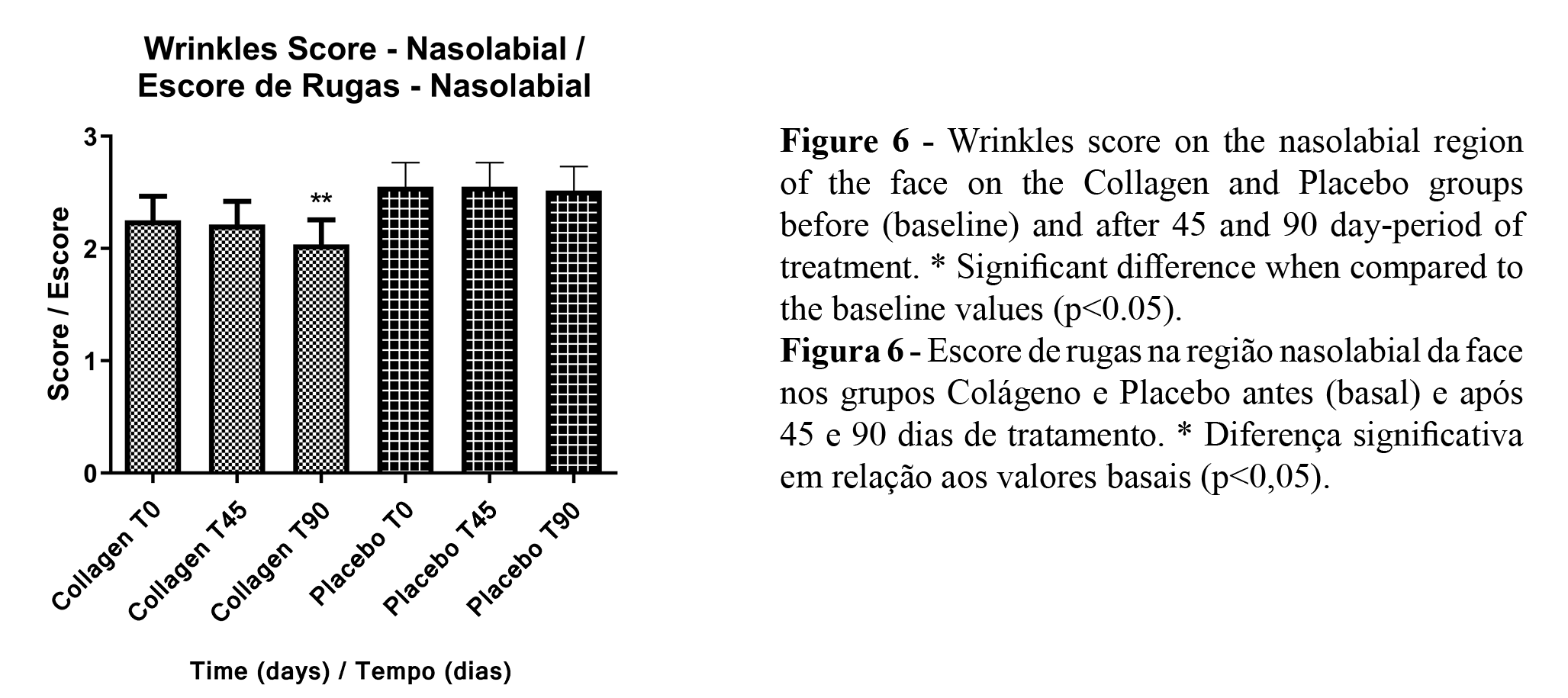
The high-resolution images obtained allowed the analysis through the score method, which presented a significant reduction of skin wrinkles in the periorbital and nasolabial regions of the face in the collagen peptides group only (Figures 5 and 6).
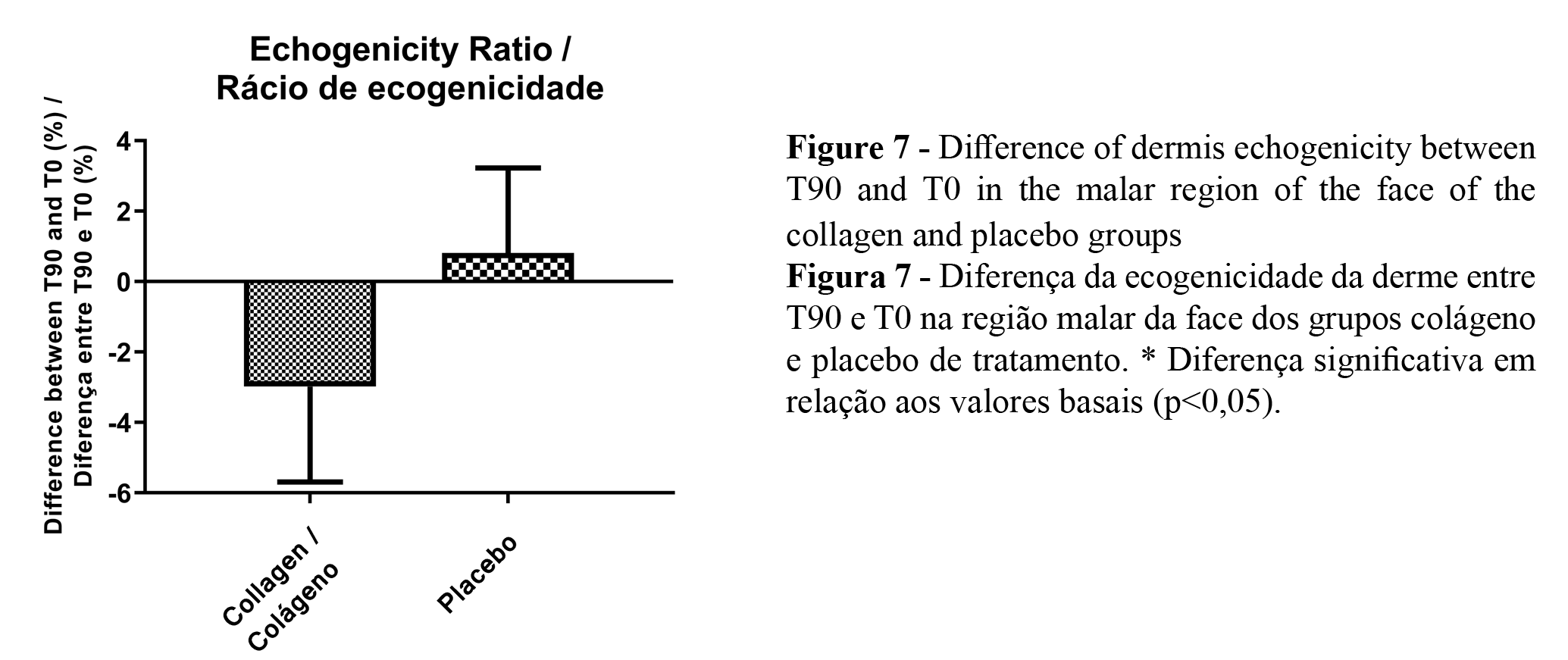
The dermis echogenicity analysis showed a decrease in the dermis echogenicity ratio (number of low echogenic pixels/number of total echogenic pixels - LEP/TEP), in both groups, after the 90 treatment period.. Figure 7 shows the echogenicity difference percentage between T90 and T0. According to these results, there was a reduction in the echogenicity ratio, therefore, an increase in the dermis echogenicity in the collagen peptides group.
Hair Characterization Studies
Tensile Test
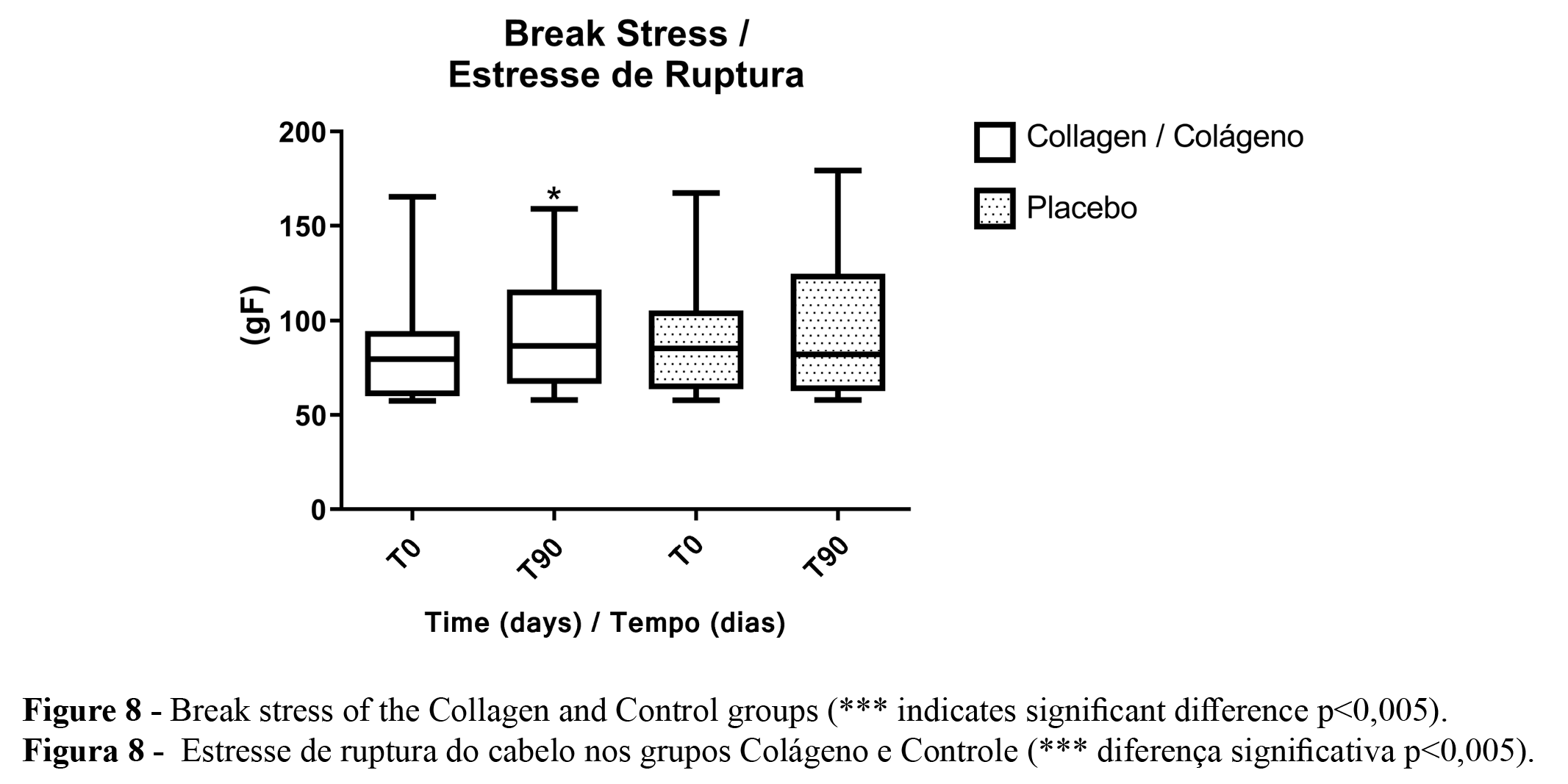
The tensile test (Figure 8) results showed that after the treatment with collagen peptides, the break stress values increased significantly. At the same time, the break stress values of the participant’s hair in the control group were unaltered.
Discussion
Collagen is one of the most abundant proteins in the human body. It is known that collagen fibers become damaged with the aging process and consequently lose their strength, causing alterations in different structures, such as the appearance of wrinkles in the skin.
Some diets can influence the skin and hair. For example, malnutrition, like in cases of anorexia nervosa, can lead to skin changes like xerosis, hair effluvium, nail modifications, etc. (26). On the other hand, obesity can also impair skin physiology. Obese people can significantly increase transepidermal water loss, which could alter skin barrier function. It can also affect sebum production, micro and macrocirculation changes, and modify collagen metabolism.
In some studies, a higher acne lesion count was correlated with the increased ratio of saturated to monounsaturated fatty acids of skin triglycerides.
An interesting study by Tanaka et al. has recently evaluated the effect of a vegetarian diet as an alternative therapy for managing Atopic dermatitis. Other studies show that it can also benefit psoriasis (26).
Furthermore, there is an association between sugar and some food processing methods (like grilling, frying, baking, etc.) with skin aging, as there are mechanisms related to advanced skin glycation end products. A high-sugar diet, ultraviolet irradiation, and eating barbecued fried foods can lead to the accumulation of AGEs and acceleration of skin aging (27).
Other studies show that strict control of blood sugar can also be significant for skin health, as four months on this diet can reduce the production of glycosylated collagen by 25%, and low-sugar food prepared by boiling can also reduce the production of AGEs (27).
There are many other effects of food on the skin, but in general reasonable, healthy, and diverse diet with antioxidant-rich foods is essential to maintaining skin health (27).
Most studies utilizing collagen peptides use a dosage of 10 g. However, the efficacy of a lower dose of the same product, without any additives as vitamins and minerals, is still to be reinforced (28). A lower dose with the same efficacy is practical to the consumer, facilitating the ingestion and creating a consumption habit with lower costs. Furthermore, with a lower dose is also easier to introduce the substance in its full dose in other finished products, creating different forms of use.
A recent systematic review and meta-analysis studies utilized a total of 1,125 patients in different studies. A comparative analysis between the placebo and the intervention group showed no difference in the means for elasticity, wrinkles, and transepidermal water loss. There was a significant difference in the dermis density, dermis thickness, and stratum corneum water content (29).
In our study, using specific imaging techniques, we obtained the same improvement in the dermis density, but, likely due to the different methods used, a difference in skin texture and wrinkles was also observed.
This way, the present study was developed to analyze the clinical effects of the consumption of 5 g of collagen peptides, without adding other components, to skin and hair.
The skin microrelief analysis showed an improvement in skin texture only in the collagen peptides study group. This improvement was explained due to changes in the protein turnover in the dermal layer; increasing the amount of collagen and the enhanced fibrillar network leads to better skin integrity and, consequently, improved skin texture. Previous studies also found this result with higher doses of collagen or products added with other substances (1,30). Similar studies have also noted changes in skin dryness, which is directly related to the skin desquamation parameter. Hydrolyzed Collagen ingestion is also known for improving skin hydration, which is inversely associated with the Sesc. Furthermore, changes in the dermal tissue, which is filled with fibroblasts, are stimulated by collagen peptides, producing new collagen, elastin, and hyaluronic acid (30). This shows that a lower dose of collagen containing no other components is effective in improving skin microrelief parameters. As it is more accessible to consumer ingestion, the food industry might develop products using this dose. The other parameters were not significant, likely because of the differences between participants.
High-resolution images provide many parameters in the study of clinical trials. It was possible to observe an improvement in skin wrinkles (in different regions) with the collagen treatment using score analysis. Beyond the mechanism described above, that also improved the skin microrelief efficiently absorbed collagen and can induce an increase of collagen fibers density and diameter (3). The increase in dermis density leads to a better structuration of the skin, with the improvement of the dermal-epidermal junction. These changes can be seen as fewer and more superficial skin wrinkles and pores (1,14).
This increase in dermis density can be confirmed by the dermis echogenicity parameter, done by a high-frequency ultrasound, which presented an improvement after 90 days of treatment with the study product, showing quantitatively and qualitatively that this increase is present. This way, the dermis echogenicity improvement directly relates to the previous parameters in this study since they were consequences of a better dermis density. A denser dermis repairs the present skin damage from different sources, slowing the chronological aging and photoaging process. In addition, the increase of dermis density found in this study with a consumption of 5 g of collagen peptides without the addition of other components per day is also in accordance with previous studies of our research group (1) and others from the literature (16,30)
Collagen is composed of peptides of several sizes, which are degraded into biologically actives di and tri peptides (30) composed of amino acids that, through different types of interactions, resulting in a more complex structure that is present in several tissues in the human body. In this context, considering that the hair fiber is also composed of a majority of proteins, mainly keratin (31), which resulted in the complexation of 20 amino acids that are also common to the collagen amino acids, so the supplementation of collagen peptides can contribute as support of amino acids for the hair fiber development in the hair bulb. After breaking the collagen molecule into less complex structures, these amino acids can contribute to the cellular development of the skin and its appendixes as the hair and nails structure. The loss of proteins in the hair fiber can reduce its resistance to mechanical and chemical processes, to which women are exposed frequently (20).
The hair is an appendage from the epidermis and can be divided into two parts, the hair follicle, and the hair shaft. The hair shaft extends from the root or bulb, which is localized in the follicle passing through the epidermis, and stratum corneum and then continuing with steam (31).
Although the hair is considered a dead structure, the region of the bulb localized in the human hair follicle is considered a complex mini-organ which allows the hair to receive nutrients and proteins that are ingested in our diet. Thus, the use of collagen peptides as a dietary supplement or in cosmetic formulations can help the protein restitution on the hair fiber and increase its strength in mechanical processes.
The results of our study show that using collagen peptides as a dietary supplement improved hair mechanical resistance since they promoted an increase in hair fiber Break Stress. This parameter evaluates the hair's internal structure and the cortex region's strength. This increase suggests that the use of collagen peptides orally promoted the treatment of the inner region (cortex) of the hair fiber, increasing its strength of the hair fiber (32). This way, collagen oral supplementation is important not only for improving skin conditions but also for hair care once significantly an increase of the mechanical hair resistance evaluated by objective measurements is noted. In addition, considering that the aging process affects the hair's mechanical resistance due to hair fiber thinning, the proposed treatment was effective for aged hair.
In summary, the present study, conducted for the time in Brazilian woman, showed important benefits in relevant skin visual parameters, dermis density, and hair strength with a lower dose of 5 g per day of bovine collagen peptides without the addition of other components.
Finally, oral supplementation with collagen peptides is important not only for the improvement of skin conditions but also for hair care once it significantly increases the mechanical hair resistance evaluated by objective measurements. In addition, considering that the aging process affects the hair's mechanical resistance due to hair fiber thinning, the proposed treatment was effective for aged hair.
Study Limitations
The limitation of the study was the difficulty of controlling and monitoring the study participants' diet, mainly in terms of protein intake. Since the aim of the study was oral supplementation, this could be influenced by the participants' dietary habits. In addition, it was not possible to quantitatively analyze the amount of white hair in the participants.
Author Contributions
P.M.B.G Maia Campos: Conceptualization, Funding acquisition, Formal analysis, Methods, Project administration, Research Supervision, Validation, Visualization, Writing - review & editing. M.O. Melo: Formal analysis, methods, writing the original draft, review and editing the text. M.M.F. Shirata: Formal analysis, methods, writing the original draft. M.G.Leite: Formal analysis, writing the original draft, methods.
Acknowledgments
We would like to thank FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant/Award Number: 2017/19278-0
Conflicts of interests
We confirm that no known conflicts of interest are associated with this publication. Furthermore, there has been no significant financial support for this work that could have influenced its results.
References
- Maia Campos, P.M.B.G., Melo, M.O, Calixto, L. et al. (2015). An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: a clinical placebo-controlled study. Clinical Pharmacology & Biopharmaceutics,4(3), 1-6. https://doi.org/10.4172/2167-065X.1000142
- Czajka, A., Kania, E. M., Genovese, L., Corbo, A., Merone, G., Luci, C., & Sibilla, S. (2018).Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutrition Research, 57, 97-108. https://doi.org/10.1016/j.nutres.2018.06.001.
- Sibilla, S., Godfrey, M., Brewer, S., Budh-Raja, A., Genovese, L. (2015). An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. The Open Nutraceuticals Journal,8, 29-42. https://doi.org/10.2174/1876396001508010029
- Chung, J. H., Seo, J. Y., Choi, H. R., Lee, M. K., Youn, C. S., Rhie, G., Cho, K. H., Kim, K. H., Park, K. C., & Eun, H. C. (2001). Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. The Journal of investigative dermatology, 117(5), 1218–1224. https://doi.org/10.1046/j.0022-202x.2001.01544.x.
- Liu, C., Sugita, K., Nihei, K., Yoneyama, K., & Tanaka, H. (2009).Absorption of hydroxyproline-containing peptides in vascularly perfused rat small intestine in situ. Bioscience, biotechnology, and biochemistry, 73(8), 1741–1747. https://doi.org/10.1271/bbb.90050
- Aito-Inoue, M., Lackeyram, D., Fan, M. Z., Sato, K., & Mine, Y. (2007). Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. Journal of peptide science : an official publication of the European Peptide Society, 13(7), 468–474. https://doi.org/10.1002/psc.870
- Shigemura, Y., Kubomura, D., Sato, Y., & Sato, K. (2014).Dose-dependent changes in the levels of free and peptide forms of hydroxyproline in human plasma after collagen hydrolysate ingestion. Food chemistry, 159, 328–332. https://doi.org/10.1016/j.foodchem.2014.02.091
- Kawaguchi, T., Nanbu, P. N., & Kurokawa, M. (2012). Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biological & pharmaceutical bulletin, 35(3), 422–427. https://doi.org/10.1248/bpb.35.422
- Watanabe-Kamiyama, M., Shimizu, M., Kamiyama, S., Taguchi, Y., Sone, H., Morimatsu, F., Shirakawa, H., Furukawa, Y., & Komai, M. (2010). Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. Journal of agricultural and food chemistry, 58(2), 835–841. https://doi.org/10.1021/jf9031487.
- León-López, A., Morales-Peñaloza, A., Martínez-Juárez, V.M., Vargas-Torres, A., Zeugolis, D.I., Aguirre-Álvarez, G. (2019) Hydrolyzed Collagen—Sources and Applications. Molecules24, 4031. https://doi.org/10.3390/molecules24224031
- Matsumoto, H., Ohara, H., Ito, K., Nakamura, Y., Takahashi, S. (2006) Clinical effects of fish type I collagen hydrolysate on skin properties. ITE Letters on Batteries, New Technologies, and Medicine, 7 (4): 386-390
- Zague, V., do Amaral, J. B., Rezende Teixeira, P., de Oliveira Niero, E. L., Lauand, C., & Machado-Santelli, G. M. (2018). Collagen peptides modulate the metabolism of extracellular matrix by human dermal fibroblasts derived from sun-protected and sun-exposed body sites. Cell biology international, 42(1), 95–104. https://doi.org/10.1002/cbin.10872
- Chen, T., Hou, H. (2016). Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth factor-β/Smad signaling pathway. Journal of Photochemistry & Photobiology, B: Biology, 162: 633–640.
- Maia Campos, P.M.B.G., Franco, R.S.B., Kakuda, L., Cadioli, G.F., Costa, G.M.D., Bouvret, E. (2012). Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study.Molecules. 26 (16):4880.
- Ohara, H., Ito, K., Iida, H., Matsumoto, H. (2009). Improvement in the moisture content of the stratum corneum following 4 weeks of collagen hydrolysate ingestion. Food Science and Technology Research, 56 (3): 137-145.
- Asserin, J., Lati, E., Shioya, T., & Prawitt, J. (2015). The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. Journal of cosmetic dermatology, 14(4), 291–301. https://doi.org/10.1111/jocd.12174
- Inoue, N., Sugihara, F., & Wang, X. (2016).Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. Journal of the science of food and agriculture, 96(12), 4077–4081. https://doi.org/10.1002/jsfa.7606.
- Proksch, E., Segger, D., Degwert, J., Schunck, M., Zague, V., & Oesser, S. (2014). Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin pharmacology and physiology, 27(1), 47–55. https://doi.org/10.1159/000351376
- Aguirre-Cruz, G., León-López, A., Cruz-Gómez, V., Jiménez-Alvarado, R., & Aguirre-Álvarez, G. (2020).Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants (Basel, Switzerland), 9(2), 181. https://doi.org/10.3390/antiox9020181
- Avila Rodríguez, M. I., Rodríguez Barroso, L. G., & Sánchez, M. L. (2018).Collagen: A review on its sources and potential cosmetic applications. Journal of cosmetic dermatology, 17(1), 20–26. https://doi.org/10.1111/jocd.12450
- Camacho-Bragado, G. A., Balooch, G., Dixon-Parks, F., Porter, C., & Bryant, H. (2015). Understanding breakage in curly hair. The British journal of dermatology, 173 Suppl 2, 10–16. https://doi.org/10.1111/bjd.13241
- Aguirre, A., Gil-Quintana, E., Fenaux, M., Erdozain, S., & Sarria, I. (2017).Beneficial Effects of Oral Supplementation With Ovoderm on Human Skin Physiology: Two Pilot Studies. Journal of dietary supplements, 14(6), 706–714. https://doi.org/10.1080/19390211.2017.1310781
- Hexsel, D., Zague, V., Schunck, M., Siega, C., Camozzato, F. O., & Oesser, S. (2017). Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. Journal of cosmetic dermatology, 16(4), 520–526. https://doi.org/10.1111/jocd.12393
- de Melo, M. O., & Maia Campos, P. M. B. G. (2018).Characterization of oily mature skin by biophysical and skin imaging techniques. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 24(3), 386–395. https://doi.org/10.1111/srt.12441
- Velasco, M.V.R., Dias, T.C., Freitas, A.Z., Vieira, N.D., Pinto, C.A.S.O., Kaneko, T.M., Baby, A.R. (2009). Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Brazilian Journal of Pharmaceutical Sciences, 41(1): 153-162.
- Piccardi, N., & Manissier, P. (2009). Nutrition and nutritional supplementation: Impact on skin health and beauty. Dermato-endocrinology, 1(5), 271–274. https://doi.org/10.4161/derm.1.5.9706
- Cao, C., Xiao, Z., Wu, Y., Ge, C. (2020).Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients, 12, 870. https://doi.org/10.3390/nu12030870
- Sangsuwan, W., & Asawanonda, P. (2021). Four-weeks daily intake of oral collagen hydrolysate results in improved skin elasticity, especially in sun-exposed areas: a randomized, double-blind, placebo-controlled trial. The Journal of dermatological treatment, 32(8), 991–996. https://doi.org/10.1080/09546634.2020.1725412
- de Miranda, R. B., Weimer, P., & Rossi, R. C. (2021). Effects of hydrolyzed collagen supplementation on skin aging: a systematic review and meta‐analysis. International Journal of Dermatology,60(12), 1449–1461. https://doi.org/10.1111/ijd.15518
- Borumand, M., Sibilla, S. (2015). Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration, and wrinkles. Journal of Medical Nutrition & Nutraceuticals, 4(1), 47-53.https://doi.org/10.4103/2278-019X.146161
- Miranda-Vilela, A. L., Botelho, A. J., & Muehlmann, L. A. (2014). An overview of chemical straightening of human hair: technical aspects, potential risks to hair fibre and health and legal issues. International journal of cosmetic science, 36(1), 2–11. https://doi.org/10.1111/ics.12093.
- Evans, T. (2013). Measuring Hair Strength, Part I: StressStrain Curves. Cosmetics and Toiletries, 128(8),1-5.
