| Original Article, Biomed Biopharm Res., 2022; 19(1):133-152 doi: 10.19277/bbr.19.1.279; pdf version [+]; Portuguese html version [PT] |
Serious Adverse Reactions to COVID-19 vaccines in Portugal up to July 2021: thrombosis with thrombocytopenia syndrome, myocarditis/pericarditis, and Guillain-Barré syndrome
Carla Pires
CBIOS – Universidade Lusófona’s Research Center for Biosciences & Health Technologies, Campo Grande 376, 1749-024 Lisboa, Portugal
corresponding author:
Abstract
Serious adverse reactions to COVID-19 vaccines may be related to death or other serious clinical events. The aim of this study was to describe and to analyze the profile of serious notified Adverse Drug Reactions (ADRs) and the occurrence of some rare and serious adverse reactions to COVID-19 vaccines in Portugal up to 22 July 2021. Data was collected from public reports of INFARMED, I.P. (the Portuguese medicine agency). Up to 22 July 2021, there were 11,002,983 administered COVID-19 vaccines in Portugal, with 11,314 notified ADRs: 36% serious and 64% non-serious. Overall, 1 ADR and 0.4 serious ADRs were reported per each 1,000 administered vaccines, respectively. There were 2 confirmed cases of thrombosis with thrombocytopenia syndrome (TTS), 3 probable cases of Guillain-Barré syndrome (GBS), and 2 definitive cases of myocarditis/pericarditis, among the studied rare serious ADRs. In conclusion, as expected, rare cases of TTS, GBS and myocarditis/pericarditis were reported, which contribute to the maintenance of a safety profile of COVID-19 vaccines at national and international levels.
Keywords: COVID-19 vaccines; thrombosis with thrombocytopenia syndrome; Guillain-Barré syndrome; myocarditis; pericarditis
Received: 08/02/2022; Accepted: 02/05/2022
Introduction
The World Health Organization (WHO) declared the COVID-19 pandemic on 11 March 2020, after the first description of 41 patients with a novel viral pneumonia in Wuhan, China on 24 January 2020 (1). The number of cases of COVID-19 reported to the WHO quickly increased. According to the WHO Coronavirus disease (COVID-19) Dashboard (8 April 2022), there have been 494,587,638 confirmed cases of COVID-19, including 6,170,283 deaths reported to the WHO, and a total of 11,250,782,214 vaccine doses have been administered globally (2). As of 10 March 2022, 3,380,263 people already have had COVID-19 in Portugal, with 21,285 (COVID-related) deaths since the beginning of the pandemic (3).
Impressively, COVID-19 vaccines were developed in a short timeframe since the beginning of the pandemic, with these medicinal products representing a determinant weapon in the mitigation of pandemic (4). Currently, there are five vaccines against COVID-19 authorized for use in the European Union (EU): Comirnaty (BioNTech and Pfizer); Nuvaxovid (Novavax), Spikevax (Moderna); Vaxzevria (previously COVID-19 Vaccine AstraZeneca), and COVID-19 vaccine Janssen (Janssen-Cilag International NV) (5). The safety and efficacy of COVID-19 vaccines are essential for their successful/rational use and for the mitigation of the pandemic (4, 6-7). Adverse drug reactions (ADRs) of COVID-19 vaccines or other medicinal products need to be closely monitored to prove there are no alterations in their safety profile and to maintain their approval status. Regulators are required to continuously demonstrate the effectiveness and safety profile of COVID-19 vaccines. The potential benefits of COVID-19 vaccines must outweigh the known risks (8).
Serious adverse events are incidents that may result in death, are life-threatening, require in-patient hospitalization or prolongation of existing hospitalization, result in persistent or significant disability/incapacity, or result in a congenital anomaly/birth defect (9-10). Any medical event that requires intervention to prevent one of the consequences of serious adverse events may also be considered as serious. However, adverse events following immunization of vaccines are predominantly categorized as non-serious: “an event that is not ‘serious’ and does not pose a potential risk to the health of the recipient” (9). Non-serious adverse events following immunization should also be carefully monitored because they may signal a potentially serious problem with the vaccine or vaccination or have an impact on the acceptability of the vaccination (9, 11). The fear of ADRs from COVID-19 vaccines is a relevant factor of subjects’ vaccine hesitancy, which may contribute to compromise the achievement of herd immunity and, consequently, the mitigation of the COVID-19 pandemic. For instance, the provision of online, written, or oral information about the efficacy and safety (e.g., ADRs) of COVID-19 vaccines may contribute to promote adherence and reduce subjects’ vaccine hesitancy (12). In Portugal, the share of vaccinated people against COVID-19 was 95% on 7 April 2022, which support very low rates of vaccine hesitancy at a national level. Portugal was the second country at a global level with the highest rate of vaccinated people, after United Arab Emirates (13).
In the EU as well as in the United States of America (USA), citizens may self-report adverse drug reactions (ADRs) online (14-15). However, ADRs have been underreported in some countries. For instance, a small number of post-vaccination adverse reactions were reported to the Sanitary Inspection of Poland in a study using a post-vaccination questionnaire (1,678 complete responses received) (14). The USA's Vaccine Adverse Event Reporting System (VAERS) accepts reports from healthcare providers, vaccine manufacturers, and the public, in a similar manner as in the EU pharmacovigilance system (15).
During the first month of COVID-19 vaccine Safety Monitoring in the USA, 14 December 2020 – 13 January 2021, only 9.2% of reports were classified as serious with the Pfizer-BioNTech and Moderna vaccines. In this timeframe, there were 6,354 (90.8%) non-serious ADRs reports, and 113 (1.6%) deaths potentially related to these two vaccines, which support their favourable safety profile (15).
The serious adverse reactions to COVID-19 vaccines currently under safety monitoring in many countries are thrombosis with thrombocytopenia syndrome (TTS), myocarditis/pericarditis, and GBS, among others (16-17). As examples, the European Medicines Agency (EMA), the "Autoridade Nacional do Medicamento e Produtos de Saúde, I.P." (INFARMED, IP; Portugal), the Therapeutic Goods Administration (TGA; Australia) and the Food and Drug Administration (FDA; USA) are closely monitoring these ADRs (16-21). These three rare and serious ADRs were also identified as relevant by several other studies (22-23). Thus, a brief explanation on TTS, myocarditis/pericarditis and GBS is presented below.
Thrombosis with thrombocytopenia syndrome
TTS is characterized by thrombosis in atypical locations (with a predominance of cerebral venous thrombosis, splanchnic vein thrombosis, or arterial thrombosis) and serious thrombocytopenia, without previous exposure to heparin (16-17). TTS is a very rare (and potentially fatal) event following vaccination with Vaxzevria and the Janssen COVID-19 vaccine, which may occur up to 30 days after vaccination (16-17, 24). The pathophysiological mechanism of TTS occurrence is not fully known, although it is thought that TTS may be explained by a similar mechanism to the immune response occasionally seen in some patients treated with heparin, i.e., the autoimmune heparin-induced thrombocytopenia (aHIT) (16, 25).
As an example, 31 cases of cerebral venous sinus thrombosis out of approximately 2.2 million doses of COVID-19 Vaccine AstraZeneca were identified by the German Society of Thrombosis and Haemostasis (4–16 days post vaccination). However, concomitant thrombocytopenia was only reported in 19 patients, with 9 fatal cases (26-27). Diverse cases of thrombosis with thrombocytopenia, including cerebral venous thromboses (CVT) were reported in the months after subjects´ immunization with adenovirus-vector COVID-19 vaccines ChAdOx1 nCOV-19 or COVID-19 Vaccine AstraZeneca, AZD1222 (Vaxzevria) and Ad26.COV2.S Janssen (Janssen-Cilag International NV) (28-30).
In addition to the recommended updates in the product information (e.g., package leaflet of medicines and summary of product characteristics, or SPC) for both Vaxzevria and the Janssen COVID-19 vaccine, healthcare professionals in the EU were informed of the signs and symptoms of TTS to facilitate its diagnosis and treatment as soon as possible, with the development and application of appropriate clinical guidelines (24).
Myocarditis/pericarditis
Myocarditis and pericarditis are inflammatory conditions of the heart, which may lead to breathlessness, alterations in heartbeat (e.g., palpitations), and/or chest pain (31). Overall, COVID-19 vaccination reduces the relative risk of myocarditis and arrhythmia manifold (32). However, there is an association between myocarditis/pericarditis and COVID-19 mRNA vaccines in children and younger adults (with males affected approximately 5-10 times more often than females), which should be carefully monitored by healthcare professionals. With the second dose of mRNA vaccine, 12.6 cases of myocarditis/pericarditis per million were reported within 21 days of vaccination in individuals aged 12 to 39 years (33-35). In general, these types of cases tend to be mild (33).
The pathophysiological mechanism of the adverse reaction myocarditis/pericarditis is also not fully known. Different mechanisms have been proposed, such as, (i) the mRNA in the vaccine may be detected as an antigen by the immune system, which may promote the activation of pro-inflammatory cascades and immunological pathways in the heart; (ii) cross-reactions between antibodies directed to SARS-CoV-2 spike glycoproteins and structurally similar human protein sequences (e.g., myocardial α-myosin heavy chain); or (iii) testosterone may be associated with the inhibition of anti-inflammatory immune cells and promotion of more aggressive T helper 1 cell-type immune responses (36).
Both the EMA and FDA updated the Product Information for the mRNA vaccines (Comirnaty and Spikevax), regarding the risk of myocarditis/pericarditis as well as developed communication materials, with instructions/guidance following vaccination with mRNA COVID-19 vaccines (21, 31, 37).
Guillain-Barré syndrome
GBS is a serious and rare autoimmune disorder of the peripheral nervous system (the body’s immune system attacks nerve cells), which typically develops within 4 weeks of an infection, such as influenza, cytomegalovirus, and glandular fever, or gastroenteritis caused by Campylobacter jejuni bacteria (22, 38-39). Particularly, a possible causal relationship between the Janssen COVID-19 vaccine and GBS was communicated by the Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA (20). As a pathophysiological mechanism, it has been hypothesized that an immune response following vaccination may produce an autoimmune process, which can lead to the production of autoantibodies against myelin (32)
Vaccination may indirectly contribute to reduce the risk of developing GBS, as vaccination decreases the risk of infectious diseases resulting from immunization. In one study, the onset of GBS after COVID-19 infection was reported for 36 patients, with a median interval of 11.5 days (22). However, a longer interval (24.2 days) was reported in another study (30 patient) (38). The number of cases of GBS after COVID-19 vaccination is rare (20, 22, 38).
Thus, the study aim was to describe and to analyze the profile of serious notified ADRs and the occurrence of some rare and serious adverse reactions to COVID-19 vaccines in Portugal up to 22 July 2021. Thus, the following research questions have been defined, as follows:
What was the profile of serious notified ADR?
- What was the ADR profile for Thrombosis with thrombocytopenia syndrome?
- What was the ADR profile for Guillain-Barré syndrome?
- What was the ADR profile for myocarditis/pericarditis?
Materials and Methods
Definition of ADR
ADRs are a “response to a medicinal product which is noxious and unintended” according to Directive 2001/83/EC of the European Parliament and of the Council (DIR 2001/83/EC Art 1(11)) (40-41). This definition is followed by INFARMED, I.P. and the EMA. The following synonyms of adverse reactions were used in the present study: adverse drug reaction (ADR) or adverse reaction.
Administered COVID-19 vaccines in Portugal and data sources
In July 2021, only four vaccines against COVID-19 were available in Portugal, specifically: Comirnaty, BNT162b2 (Pfizer-BioNTech) and Spikevax, mRNA-1273 (Moderna) (two mRNA-based vaccines), and Vaxzevria (AZD1222) and Janssen, Ad26.COV2.S (Janssen-Cilag). These vaccines were approved by the EMA through a centralized approval procedure and are used in the EU (5, 42).
ADRs data potentially related to COVID-19 vaccines up to 22 July 2021 in Portugal were collected in two publications/reports from INFARMED, I.P. (the Portuguese medicine agency) (16-17). It should be noted that pediatric vaccination only began in Portugal on 18 December 2021 (43).
Self-reports of ADRs in Portugal
In Portugal, the reporting of suspected ADRs may be made online (https://www.infarmed.pt/web/infarmed/portalram) (44). Four elements are obligatory collected: the suspected ADR(s); the name(s) of likely related medicine(s); patient data (such as initials or age or sex); and the means of contact of the notifier of the ADR. Collected data are validated by experts (pharmacists and physicians) (44). These data are sent to two international databases of ADRs: the European (EudraVigilance) and worldwide WHO (VigiBase) ADR databases aiming at following the safety profile of medicines (45). Reports of ADRs may be carried out by a health professional and/or a citizen/patient, and if at least one ADR is classified as serious, the case is also classified as serious (9, 16-17).
Classification of serious ADRs
Serious ADRs were classified according to the WHO classification as follows: disability; hospitalization; congenital anomaly; life threatening, death or other (clinically relevant) (9, 16-17). This classification is also adopted by INFARMED, I.P. and the EMA (16-17, 46-47). ADRs are presented according to INFARMED,I.P. reports (9, 16-17).
Selected rare and serious ADRs: applied classification criteria
TTS, myocarditis/pericarditis, and GBS were purposely selected, since these three rare and serious ADRs are under close attention at both national and international levels according to the Pharmacovigilance report from INFARMED, I.P. on the monitorization of the safety of COVID-19 vaccines in Portugal up to 22 July 2021 (16-17).
Both the INFARMED, I.P. and the EMA follow the criteria of the Brighton Collaboration to classify TTS, myocarditis/pericarditis, and GBS (16-17, 48-50). According to this classification, a case of TTS is classified as confirmed when the following cumulative factors occur thrombosis in an atypical location; platelet count <150 x109 per L; D-Dimers >4,000 ng/mL; and anti-PF4+ antibodies. A case of TTS is classified as probable when the following cumulative factors occur thrombosis in an atypical location; platelet count <150 x109 per L; and D-Dimers >4,000 ng/mL. A case of TTS is classified as possible when the following cumulative factors occur: thrombosis in an atypical location and platelet count <150 x109 per L) (16-17, 48). Myocarditis/pericarditis were classified, as follows: level 1 (definitive), level 2 (probable), level 3 (possible), level 4, and level 5 (16-17, 49). Finally, GBS was classified in five levels according to Decision tree algorithm for GBS and Miller Fisher Syndromes Level of Diagnostic Certainty (Brighton collaboration case definition): level 1 (definitive), level 2 (probable), level 3 (possible), level 4, and level 5 (50).
Important remarks
The report of a certain suspected ADR does not necessarily presuppose the existence of a causal relationship between the reported adverse reaction and a certain medication, according to the information from diverse medicine agencies, such as the EMA or INFARMED, I.P. (16-17, 51-52).
Results and Discussion
Serious ADRs
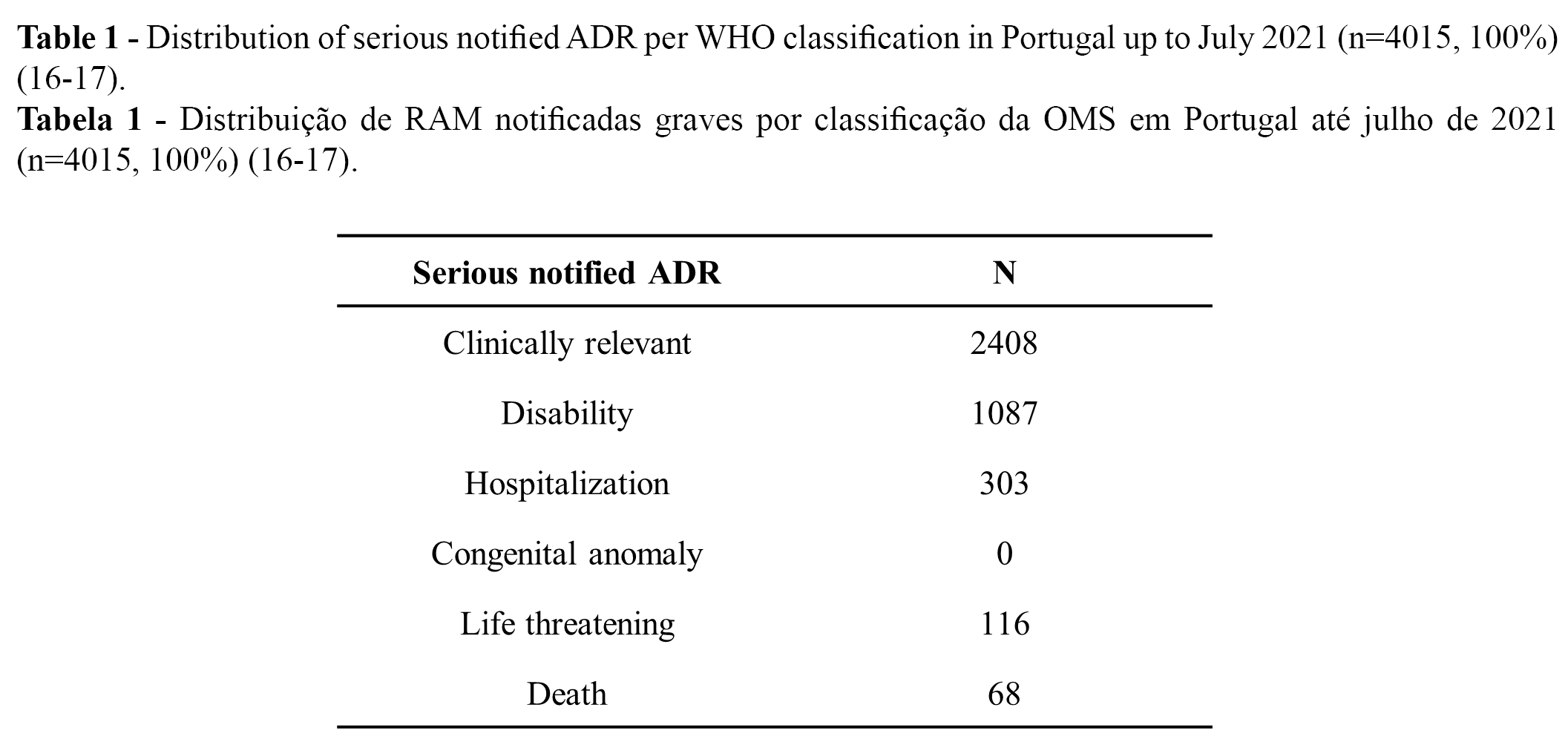
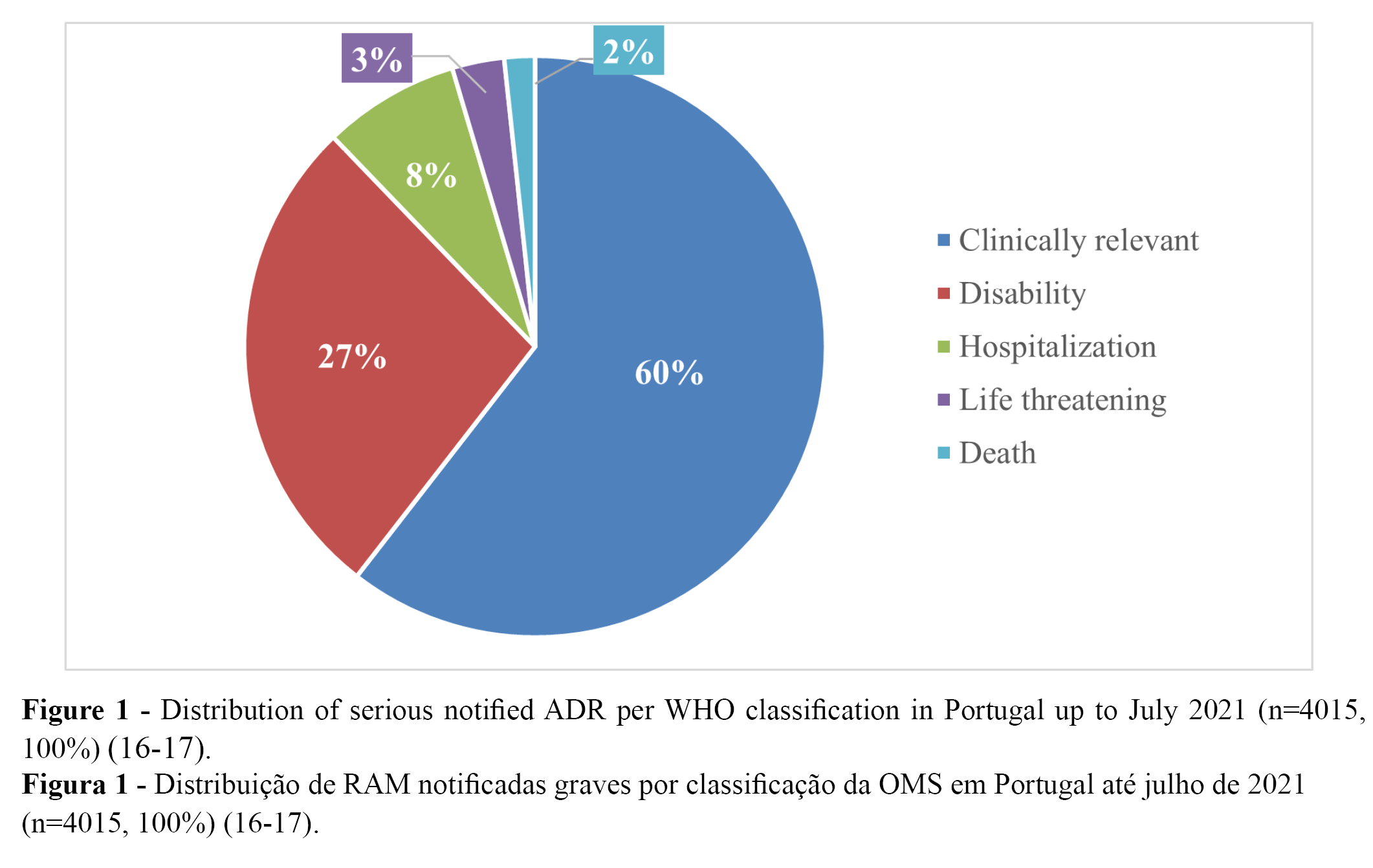
From the 11,314 notified ADRs (100%), 36% (n = 4015) were classified as serious, while 64% were classified as not serious (16-17). Overall, more than 90% of all serious cases (n = 4,015) were related to temporary incapacity (e.g., absenteeism). Regarding the distribution of the serious notified ADRs, 9,6% (n = 1,087) were classified as “leading to disability”, and 0.6% as “may have led to death” (16-17). The distribution of serious notified ADRs according to WHO classification is presented in Table 1 (n) and Figure 1 (%).
Reports associated with serious ADRs: TTS, GBS, and myocarditis/pericarditis
Thrombosis with thrombocytopenia syndrome
In Portugal, up to 22 July 2021 (cumulative number of all COVID-19 vaccines = 11,002,983), 7 cases of TTS were identified, all related with the vaccine Vaxzevria, as follows (16-17):
- Confirmed cases of TTS (n = 2);
- Probable cases of TTS (n = 2);
- Possible cases of TTS (n = 3).
Guillain-Barré syndrome
In Portugal, up to 22 July 2021 (cumulative number of all COVID-19 vaccines = 11,002,983), 13 cases of GBS potentially related to COVID-19 vaccination were identified: 11 cases of GBS potentially related to the vaccine Vaxzevria (3 cases from level 2 (probable) and 8 cases from level 4) and 2 cases of GBS with the Janssen vaccine (1 case from level 1 (definitive) and 1 case from level 4) (16-17).
Myocarditis/pericarditis
Overall, 9 cases of myocarditis/pericarditis were identified in Portugal up to 22 July 2021. All cases were related to Comirnaty and occurred in subjects who were more than 30 years of age (16-17).
Discussion
The discussion is organized in four sections: one section on severity and classification of ADRs and three sections on the studied serious and rare ADRs, respectively: TTS, GBS, and myocarditis/pericarditis. The reply to the four defined research questions is addressed and discussed in these sections.
Severity and classification of ADRs
Serious adverse events to COVID-19 vaccines are rare (i.e., may affect up to 1 in 1,000 people) (23, 53). Positively, only around one third from all reports of ADRs were classified as serious, and more than 90% from all serious cases were related to a situation of temporary incapacity, which support a favorable safety profile of COVID-19 vaccines in Portugal. ADRs reports from the EU (EudraVigilance database) present a similar profile, with most cases of suspected ADRs being associated with non-serious adverse reactions (e.g., ‘flu-like’ illness, headache, pain at the application site, chills, fatigue, nausea, fever, dizziness, weakness, myalgia, and tachycardia) (38).
In Portugal, deaths after COVID-19 vaccination have occurred in subjects with a median age of 78 years. These deaths may not be directly related with the administration of these vaccines. For instance, fatalities may be explained by the natural patterns of morbidity or mortality of the Portuguese population (68 deaths per 11002983 administered vaccines in Portugal up to July 2021) (16-17). The reported deaths to the TGA after COVID-19 vaccination in Australia (810 reports of deaths received and reviewed in about 56.2 million administered doses of COVID-19 vaccines up to 31-3-2022) were proportionally higher than in Portugal. However, TGA only linked death to COVID-19 vaccination in 11 cases (people aged 34-81 years old) (18).
The percentage of reported deaths in the present study was also lower than the percentage reported in Gee et al. (2020) (15), i.e., 0.6% vs. 1.6%, which may be explained by different populational characteristics or different percentages of self-reports. It is possible that some ADRs may have not been notified/reported by citizens in Portugal, for instance due to the lack of knowledge of some patients/citizens about how to report an ADR online. Further studies are recommended on the present topic, such as studies to characterize the social determinants related to self-reporting of ADRs or clinical studies to characterize the pathophysiological mechanism of serious and rare or potential fatal ADRs.
Reports related to serious and rare ADRs: TTS, GBS, and myocarditis/pericarditis
An adverse event after vaccination is defined as “any untoward medical event that follows immunization and that does not necessarily have a causal relationship with the usage of the vaccine” (9). Thus, adverse reactions to COVID-19 vaccines (serious and non-serious) must be closely monitored through self-reports, along with additional clinical and/or pharmacoepidemiologic studies (if necessary) to monitor their safety and efficacy profile.
Additionally, it is expected that all EU member states follow the recommendations of the EMA, regarding the analysis/classification of the reports of ADRs such as TTS, GBS, and myocarditis/pericarditis. Reports of suspected ADRs are sent by medical agencies, such as INFARMED, I.P., to EudraVigilance database, which is “the system for managing and analyzing information on suspected adverse reactions to medicines which have been authorized or being studied in clinical trials in the European Economic Area (EEA). The EMA operates the system on behalf of the EU medicines regulatory network” (54-55). Thus, ADRs information of COVID-19 vaccines from EudraVigilance seem suitable to be compared between the member states of UE.
Thrombosis with thrombocytopenia syndrome
Diverse cases of thrombosis with thrombocytopenia, including cerebral venous thrombosis, were reported with both COVID-19 vaccines (Vaxzevria and Janssen) within the EU (56-59). For example, 11 cases of unusual thrombotic events in combination with thrombocytopenia were reported in Germany and Austria (March 2021); median age of 36 years; 9 out of 11 cases were women, and 6 of them died. These cases occurred 5-20 days after vaccination (60). Females seem to be affected more often by sinus vein thromboses associated with two of the vector-based vaccines (61). Similar clinical observations were reported for 5 cases in 130,000 vaccinated subjects in Norway (3 subjects died) (62). Particularly, up to 3 April 2022, 166 TTS (38 fatal) reports have been communicated to EudraVigilance database for Vaxzevria and 40 TTS (9 fatal) reports for Janssen vaccine, with 151,876,503 administered doses of Vaxzevria and 632,903,54 administered doses of Janssen vaccine in the EU on 7 April 2022 (54, 63).
As expected, the occurrence of TTS was very rare in Portugal: only 2 confirmed cases of TTS (all with Vaxzevria) per 11,002,983 administered COVID-19 vaccines (Vaxzevria, n = 1,141,821) up to 22 July 2021. The inexistence of confirmed cases of TTS associated with Janssen vaccines in Portugal up to July 2021 may be justified by the lower number of administered doses of this vaccine at a national level (i.e., 444,733) (16-17).
Additionally, the TGA has evaluated the reports of TTS according to similar criteria to those applied in Portugal. These criteria respectively were, as follows: INFARMED, I.P.: thrombosis in an atypical location; platelet count <150 x109 per L; D-Dimers >4,000 ng/mL; and anti-PF4+ antibodies (16-17) vs. TGA: “patients with any site of new thrombosis who have recently received vaccination against COVID (day 4-20), should be further investigated for TTS”: (1) the platelet count is <150x109/L and either (2) D-dimers are elevated (5x ULN) or (3) fibrinogen is reduced, and either positive ELISA test for anti-PF4 antibodies and functional HIT testing (64). TGA have identified about 2 cases of TTS in every 100,000 vaccinated people with Vaxzevria (after the first dose). This risk significantly decreased after the second dose (i.e., 0.3 in every 100,000 vaccinated people) (18). TGA reports of TTS for Vaxzevria totaled 112 cases classified as possible or probable (62 confirmed, 50 probable) from approximately 8.1 million vaccine doses up to 19 August 2021 (39). The confirmed cases of TTS per million Vaxzevria doses administered are proportionally higher in Australia than Portugal, although classified as rare in both territories. Discrepancies between Portugal and Australia, regarding the number of confirmed cases of TTS may have been due to differences between subjects’ genetic susceptibility, morbidities, evaluation methodology, underreporting, or other. Further studies are recommended on this topic.
Guillain-Barré syndrome
A possible a causal relationship between COVID-19 vaccine Janssen and GBS was communicated by PRAC (20, 65). Regarding the reports of GBS in the EudraVigilance database, up to 3 April 2022, 1451 reports (22 fatal) of GBS for Vaxzevria (151.876.503 administered doses of Vaxzevria, 7 April 2022) and 474 reports (3 fatal) of GBS were reported for the Janssen vaccine (63.290.354 administered doses of the Janssen vaccine, 7 April 2022) (16-17, 54). It is important to notice that some of the reported cases may eventually be explained by the natural patterns of occurrence of GBS in the population (16-17, 54). Additionally, differences between the constitution of the two vaccines may explain variations between the report rates of GBS. For instance, Vaxzevria and Janssen adenovirus vectors present substantial differences (specimen origin): the Ad26.COV2.S vaccine uses a human Ad26–based vector, whereas the ChAdOx1 nCoV-19 vaccine uses a chimpanzee adenovirus–based vector (58, 66).
In Portugal, the number of cases of GBS potentially related to the administration of a COVID-19 vaccine was higher for Vaxzevria (n = 11) (2,003,932 administered doses of Vaxzevria up to July 2021 in Portugal) than Janssen (n = 2) (444,733 administered doses of Janssen COVID-19 up to 22 July 2021) (16-17), which may be explained by the lower number of Janssen vaccines administered in Portugal up to 22 July 2021 or due to differences in the constitution of vaccines (as previously mentioned).
Overall, 310 preliminary reports of GBS were identified in VAERS (March 24, 2022), after the administration of more than 18.5 million J&J/Janssen COVID-19 vaccine doses in the USA (21). VAERS have not followed the Brighton collaboration criteria regarding the classification of GBS, because of the limited number of reported cases (13). TGA reported GBS in about one in every 100,000 people after vaccination with Vaxzevria, while Janssen vaccine was not being administered in Australia on 31-3-2022 (18). Additionally, on 9-4-2022, there was no published information in the official websites of TGA or the FDA supporting that these medicine agencies had followed the Brighton collaboration criteria to classify GBS reports. Proportionally, the reported cases of GBS in Portugal were also lower than in the USA and Australia, which eventually may be explained by underreporting of GBS after COVID-19 vaccination in Portugal, populational differences (e.g., genetic patterns, which can be associated with different susceptibilities), and/or discrepancies in the applied evaluation methodologies of ADRs.
According to the data from some regions, the number of reported cases of GBS have increased since the beginning of pandemic, with diverse reports of GBS after a COVID-19 infection (e.g., the rate per year was estimated to be 2.43/1,000,000 and 0.93/1,000,000 in 2020 and 2019, respectively, in northern Italy). On the other hand, COVID-19 vaccination may indirectly contribute to reduce the incidence of GBS, since immunization reduces the risk of infection (22, 38). For example, the risk of GBS after influenza infection or vaccination was evaluated in Ontario, Canada (1993 and 2011), with 1 GBS admission per million vaccinations being found in comparison to 17 GBS admissions per million cases of influenza (67).
PRAC has taken some regulatory actions on this topic, such as the recommendation that health professionals and vaccinated subjects (non-replicative viral vector vaccines) give special attention to the possible appearance of signs or symptoms of GBS. Particularly, the PRAC (meeting of 5-8 July 2021) recommended an update to the product information for Vaxzevria to include a warning to raise the awareness of healthcare professionals to report GBS cases following vaccination (16-17, 31). As well, GBS was listed and classified as a very rare side effect in the summary of product characteristics of the COVID-19 vaccine Janssen following PRAC recommendations (20).
Myocarditis/pericarditis
Myocarditis and pericarditis occur within 10 days after vaccination, particularly after the second dose of Comirnaty (Pfizer-BioNTech), and more often in younger men. Positively, these cardiac events are usually transient and resolve following rest, with only some patients requiring hospitalisation (39). According to the PRAC, myocarditis and pericarditis can occur in very rare cases following vaccination with Comirnaty and Spikevax (previously Moderna COVID-19 vaccine). A detailed review by PRAC, including the data from EudraVigilance database respectively found 145 cases of myocarditis and 138 cases of pericarditis in the EEA among the recipients of the Comirnaty vaccine (among approximately 177 million doses of Comirnaty have been administered in EEA, 31 May 2021) and 19 cases of myocarditis and 19 cases of pericarditis in recipients of Spikevax (among approximately 20 million doses of Spikevax have been administered in EEA, 31 May 3021) (19).
TGA have classified as likely myocarditis: 36 cases level 1, 373 cases level 2, and 100 cases level 3 for Comirnaty (Pfizer) (38.4 million doses given) and 1 case level 1, 66 cases level 2, and 13 cases level 3 for Spikevax (Moderna) (4 million doses given) up to 27 March 2022. The applied criteria by TGA to classify the likely cases of myocarditis were, as follows: “cases classified as level 1 are confirmed to be myocarditis based on strong clinical evidence including the patient's symptoms, and results of tests and imaging indicating a diagnosis of myocarditis. Level 2 cases are probably myocarditis based on a combination of symptoms and routine tests for heart conditions. Level 3 cases are possibly myocarditis based on symptoms and a doctor's report that myocarditis is the most likely diagnosis in the absence of medical tests and investigations. For all cases of suspected myocarditis, where possible, other known causes of the patient's symptoms or test results are ruled out before cases are classified” (18), i.e., the criteria of Brighton Collaboration to define myocarditis were not specifically followed by TGA.
Globally, the number of cases of myocarditis/pericarditis after COVID-19 vaccination in Portugal up to 22 July 2021 were proportionally lower than the number of reported cases in EEA and TGA, i.e., 8 cases of myocarditis/pericarditis (all with Comirnaty; 7 412 497 administered doses): 2 cases from level 1 - definitive; 1 case from level 2 - likely, and 2 cases from level 3 – possible (16-17). However, these findings are not directly comparable with other regions, which have not specifically followed the Brighton collaboration criteria in the classification of myocarditis/pericarditis (e.g., TGA in Australia). As well, these findings can indicate underreporting of ADRs by citizens and/or healthcare professionals in Portugal, populational differences (e.g., genetic profile or patterns of morbidity) and/or discrepancies in the applied evaluation methodologies of ADRs between regions.
Considering that PRAC has confirmed a possible association between the administration of mRNA vaccines and the appearance of myocarditis/pericarditis., the regulatory recommendations of PRAC were to list myocarditis and pericarditis as new adverse reactions in the product information (e.g., in the package leaflets of these vaccines) as well as to inform healthcare professionals and subjects on the present topic, for instance, to motivate them to report these ADRs (16-17, 31).
Practical implications and future research
Divulgations/campaigns on how to report ADRs in mass media and social networks can be useful to raise the awareness of the general public on the present topic, since reports of ADRs are fundamental to establish a causal relationship between ADRs and the administration of a certain medicine. The establishment of these causal relationships follow narrow scientific and regulatory criteria (e.g., generation and evaluation of signs, i.e., in pharmacovigilance a signal is defined as “information arising from one or multiple sources, including observations and experiments, which suggests a new potentially causal association, or a new aspect of a known association between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action”) (16-17). ADRs (serious and non-serious) should be narrowly and continually monitored to detect any possible modification in the patterns of ADRs at a global level.
Additionally, complementary pharmacoepidemiologic and clinical studies with a rigorous scientific design can be recommended to monitor the safety profile of COVID-19 vaccines (68), although complementary clinical studies are only proposed in situations that clearly need explanation. For instance, if the methods described above are insufficient to establish a potential association between the exposure to a particular medicine and a certain adverse reaction (69). Findings from these studies may be especially relevant to the implementation of political and public health measures (e.g., to accurately select the safest vaccines against COVID-19 in a certain region) and/or to monitor the safety of COVID-19 vaccines in the long term.
Study limitations
Comparisons of the profile of ADRs between different countries are not possible, as equivalent evaluation methodologies of ADRs may have not been followed (e.g., Brighton collaboration criteria were not specifically followed in all regions/countries). Additionally, clinical data from individual reported cases are not public (e.g., in the pharmacovigilance reports/bulletins), which necessarily limited the analyses/comparisons provided.
Conclusion
In general, the proportion of reported ADRs (serious and non-serious) was low (1 ADR and 0.4 serious ADRs per 1,000 administered COVID-19 vaccines) in Portugal up to 22 July 2021 (16-17). These findings support the maintenance of a favorable safety profile of COVID-19 vaccines at a national and international level.
The report of suspected adverse reactions per se is not enough to determine the likelihood of a certain adverse reaction and/or to prove/confirm a potential link between the medicine/vaccine and the observed adverse reaction (51-52). For instance, the occurrence of deaths or other serious reported ADRs may be explained by the normal patterns of mortality and/or morbidity rates of populations.
As expected, the reports of potentially serious ADRS after COVID-19 vaccination, namely TTS, GBS, and/or myocarditis/pericarditis were very rare at national and international levels. These three ADRs may have been underreported in Portugal in comparison to other countries, such as Australia or the USA. However, these differences may be explained by differences between populations and/or the application of different methodological approaches to evaluate ADRs.
Authors Contributions Statement
This work was fully carried out by Carla Pires (conceptualization, drafting, data analysis, and reviewing).
Funding
None.
Acknowledgements
Not applicable.
Conflict of Interests
The author declares there are no financial and/or personal relationships that could present a potential conflict of interests.
References
- Cucinotta, D., & Vanelli, M. (2020). WHO Declares COVID-19 a Pandemic. Acta bio-medica : Atenei Parmensis, 91(1), 157–160. https://doi.org/10.23750/abm.v91i1.9397
- WHO. (2022). WHO Coronavirus (COVID-19) Dashboard, 2022. Available online: https://covid19.who.int/ (accessed on 9-4-2022)
- DGS – Direção Geral de Saúde. (2022). Portuguese COVID-19 report on 3-2-2022. Available online: https://covid19.min-saude.pt/relatorio-de-situacao/(accessed on 30-4-2022).
- Carvalho, T., Krammer, F., & Iwasaki, A. (2021). The first 12 months of COVID-19: a timeline of immunological insights. Nature reviews. Immunology, 21, 245–256.
- EMA. (2021). COVID-19 vaccines: authorized, 2021. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section (accessed on 5-9-2021).
- Williams, J., Degeling, C., McVernon, J., & Dawson, A. (2021). How should we conduct pandemic vaccination? Vaccine, 39, 994–999.
- Frederiksen, L., Zhang, Y., Foged, C., & Thakur, A. (2020). The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Frontiers in immunology, 11, 1817.
- WHO. (2021). Statement for healthcare professionals: How COVID-19 vaccines are regulated for safety and effectiveness, 11 June 2021. Available online: https://www.who.int/news/item/11-06-2021-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness(accessed on 5-9-2021).
- WHO. (2020). COVID-19 vaccines: Safety Surveillance Manual, 2020. Available online: https://www.who.int/vaccine_safety/committee/Module_AEFI.pdf?ua=1 (accessed on 4-9-2021).
- FDA. (2021). What is a Serious Adverse Event? Available online: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed on 3-4-2021).
- NSW government. (2021). Adverse Event Following Immunisation control guideline. Available online: https://www.health.nsw.gov.au/Infectious/controlguideline/Pages/adverse.aspx (accessed on 4-4-2021).
- Pires C. (2021). What Is the State-of-the-Art in Clinical Trials on Vaccine Hesitancy 2015-2020? Vaccines, 9, 348.
- Our world in data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed 9-4-2022).
- Jęśkowiak, I., Wiatrak, B., Grosman-Dziewiszek, P., & Szeląg, (2021). A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines, 9, 502.
- Gee, J., Marquez, P., Su, J., et al. (2021). First Month of COVID-19 vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep,70:283–288.
- INFARMED, I.P. (2021). Pharmacovigilance Report – Monitorization of the safety of COVID-19 vaccines up to 22-7-2022. [Relatório de Farmacovigilância Monitorização da segurança das vacinas contra a COVID-19 em Portugal up to 22-7-2021].
- INFARMED, I.P. (2021). Pharmacovigilance Bulletin: monitorization of the safety of COVID-19 vaccines up to 22-7-2021. [Monitorização da segurança das vacinas contra a COVID-19 em Portugal até 22-7-2021]. V. 25, Number 5, 2021. Available online: https://www.infarmed.pt/documents/15786/4230446/Boletim+de+Farmacovigil%C3%A2ncia%2C+Volume+25%2C+n%C2%BA5%2C+maio+de+2021/fb014726-c395-b78b-d75d-34c0c37939d5?version=1.0(accessed on 23-10-2021).
- TGA. (2022). COVID-19 vaccine weekly safety report - 31-03-2022. Available online: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-31-03-2022 (accessed on 6-4-2022).
- EMA. (2021). Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis. Available online: https://www.ema.europa.eu/en/news/comirnaty-spikevax-possible-link-very-rare-cases-myocarditis-pericarditis (accessed on 6-4-2022).
- EMA. (2021). COVID-19 vaccine Janssen: Guillain-Barré syndrome listed as a very rare side effect. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-guillain-barre-syndrome-listed-very-rare-side-effect(accessed on 3-4-2021).
- Centers for Disease Control and Prevention (CDC). (2022). Selected Adverse Events Reported after COVID-19 Vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html(accessed on 3-4-2021).
- Uncini, A., Vallat, J.M., & Jacobs, B.C. (2020). Guillain-Barre´ syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry., 91, 1105–10.
- Wu, Q., Dudley, M. Z., Chen, X., Bai, X., Dong, K., Zhuang, T., Salmon, & D Yu, H. (2021). Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC medicine, 19, 173.
- EMA’s COVID-19 taskforce. (2021). EMA raises awareness of clinical care recommendations to manage suspected thrombosis with thrombocytopenia syndrome, June 2021. Available online: https://www.ema.europa.eu/en/news/ema-raises-awareness-clinical-care-recommendations-manage-suspected-thrombosis-thrombocytopenia(accessed on 11-9-2021).
- WHO. (2021). Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). Available online: https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf?sequence=1&isAllowed=y(accessed on 3-4-2021).
- Alam, W. (2021). COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A review of the potential mechanisms and proposed management. Science progress, 104, 368504211025927.
- Oldenburg, J., Klamroth, R., Langer, F., et al. (2021). Diagnosis and management of vaccine related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie, 41, 184–189.
- EMA. (2021). COVID-19 vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets, April 2021. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood(accessed on 10-9-2021).
- Thakur, K.T., Tamborska, A., Wood, G.K., McNeill, E., Roh, D., Akpan, I.J., et al. (2021). Clinical review of cerebral venous thrombosis in the context of COVID-19 vaccinations: Evaluation, management, and scientific questions. Journal of the neurological sciences, 427, 117532.
- Wise, J. (2021). Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ (Clinical research ed.), 372, n699.
- PRAC. (2021). Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 5-8 July 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-5-8-july-2021(accessed on 11-9-2021).
- Rao, S. J., Khurana, S., Murthy, G., Dawson, E. T., Jazebi, N., & Haas, C. J. (2021). A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Journal of community hospital internal medicine perspectives, 11(5), 597–600. https://doi.org/10.1080/20009666.2021.1954284.
- Luk, A., Clarke, B., Dahdah, N., Ducharme, A., Krahn, A., McCrindle, B., et al. (2021). Myocarditis and Pericarditis following COVID-19 mRNA Vaccination: Practical Considerations for Care Providers. The Canadian journal of cardiology, S0828-282X(21)00624-3.
- Shimabukuro, T. (2021). COVID-19 vaccine safety updates Advisory Committee on Immunization Practices (ACIP), 23 June 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf(accessed on 12-9-2021).
- Klein NP, Lewis N, Goddard K, et al. (2021). Surveillance for Adverse Events After COVID-19 mRNA Vaccination.JAMA. doi:10.1001/jama.2021.15072.
- Heymans, S., & Cooper, L. T. (2022). Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nature reviews. Cardiology, 19(2), 75–77. https://doi.org/10.1038/s41569-021-00662-w.
- WHO. (2021). COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated guidance regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines. Available online: https://www.who.int/news/item/09-07-2021-gacvs-guidance-myocarditis-pericarditis-covid-19-mrna-vaccines(accessed on 4-4-2021).
- Koike, H., Chiba, A., & Katsuno, M. (2021). Emerging Infection, Vaccination, and Guillain-Barré Syndrome: A Review. Neurology and therapy, 1–15.
- Therapeutic Goods Administration. (2021). COVID-19 vaccine weekly safety report - 19-08-2021. Available online: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-19-08-2021 (accessed on 12-9-2021).
- HMA & EMA. (2017). Guideline on good pharmacovigilance practices (GVP), 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf(accessed on 5-9-2021).
- FDA. (2018). Finding and Learning about Side Effects (adverse reactions), 2018. Available online: https://www.fda.gov/drugs/information-consumers-and-patients-drugs/finding-and-learning-about-side-effects-adverse-reactions(accessed on 5-9-2021).
- Francis, A. I., Ghany, S., Gilkes, T., & Umakanthan, S. (2021). Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgraduate medical journal2021, 140654. https://doi.org/10.1136/postgradmedj-2021-140654.
- Serviço Nacional de Saúde (SNS). (2022). Vaccination of children aged 5 to 11. [Vacinação de crianças dos 5 aos 11]. Available online: https://www.sns.gov.pt/noticias/2022/01/11/vacinacao-de-criancas-dos-5-aos-11/ (accessed on 5-2-2022).
- INFARMED, I.P. (2016). Notificação de reações adversas/efeitos indesejáveis de medicamentos. Available online: https://www.infarmed.pt/web/infarmed/portalram(accessed on 4-4-2021).
- Felicetti, P., Trotta, F., Bonetto, C., et al. Collaboration Vasculitis Working Group (2016). Spontaneous reports of vasculitis as an adverse event following immunization: A descriptive analysis across three international databases. Vaccine, 34(51), 6634–6640. https://doi.org/10.1016/j.vaccine.2015.09.027
- Lei n.º 21/2014 - Aprova a lei da investigação clínica. Available online: https://dre.pt/dre/detalhe/lei/21-2014-25344024(accessed on 4-4-2021).
- Directive 2001/83/EC of the European parliament and of the council of 6 November 2001 on the Community code relating to medicinal products for human use (OJ L 311, 28.11.2001, p. 67). Available online: https://ec.europa.eu/health/system/files/2016-11/dir_2001_83_cons_2012_en_0.pdf(accessed on 4-4-2021).
- Brighton Collaboration. Interim case definition of thrombosis with thrombocytopenia (TTS). Available online: https://brightoncollaboration.us/wp-content/uploads/2021/05/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf(accessed on 9-4-2022).
- Brighton Collaboration. Myocarditis/Pericarditis Case Definition. Available online: https://brightoncollaboration.us/wp-content/uploads/2021/07/BC-Myocarditis-Algorithm_-7-15-2021_FINAL.docx(accessed on 9-4-2022).
- Brighton Collaboration. GBS Case Definition. Available online: https://brightoncollaboration.us/wp-content/uploads/2021/04/GBS_Decision-Tree-Algorithm.pdf(accessed on 9-4-2022).
- EMA. (2014). Recommendation on harmonizing the approach to causality assessment for adverse events to veterinary medicinal products (revision 1), 2014. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/post-authorisation/pharmacovigilance/guidance/recommendation-harmonising-approach-causality-assessment-adverse-events-veterinary-medicinal(accessed on 12-9-2021).
- EMA. (2021). EudraVigilance: European database of suspected adverse drug reaction reports. Available online: https://www.adrreports.eu/ (accessed on 23-10-2021).
- EMA. (2022). Product-information templates – Human. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/product-information/product-information-templates-human (accessed on 5-4-2022).
- EMA. (2022). EudraVigilance database. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance(accessed on 8-4-2022).
- EMA. (2021). EudraVigilance Disclaimer. Available online: https://www.adrreports.eu/en/disclaimer.html(accessed on 8-4-2022).
- ECDC - European Centre for Disease Prevention and Control. (2021). Suspected adverse reactions to COVID19 vaccination and the safety of substances of human origin, June 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Suspected-adverse-reactions-to-COVID-19-vaccination-and-safety-of-SoHO.pdf(accessed on 23-10-2021).
- Riad, A., Pokorná, A., Mekhemar, M., Conrad, J., Klugarová, J., Koščík, M., et al. (2021). Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines,9, 673.
- Sadoff, J., Gray, G., Vandebosch, A., Cárdenas, V., Shukarev, G., Grinsztejn, B., Spiessens, B., et al. (2021). ENSEMBLE Study Group Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med., 384, 2187–2201.
- Wi, Y.M., Kim, S.H., & Peck, K.R. (2021). Early Adverse Events between mRNA and Adenovirus-Vectored COVID-19 vaccines in Healthcare Workers. Vaccines, 9, 931.
- Greinacher, A., Thiele, T., Warkentin, T.E., Weisser, K., Kyrle, P.A., & Eichinger, S. (2021). Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med, 384, 2092-2101.
- Brady, E., Nielsen, M. W., Andersen, J. P., & Oertelt-Prigione, S. (2021). Lack of consideration of sex and gender in COVID-19 clinical studies. Nature communications, 12, 4015.
- Brüssow, H. (2021). COVID-19: vaccination problems. Environmental microbiology, 23, 2878–2890.
- European Centre for Disease Prevention and Control. (2022). COVID-19 vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab(accessed on 8-4-2022).
- THANZ Advisory Statement for Haematologists. (2021). Suspected Vaccine Induced Prothrombotic Immune Thrombocytopenia (VIPIT)/Vaccine induced immune thrombotic thrombocytopenia VITT). Available online: https://www.racgp.org.au/FSDEDEV/media/documents/RACGP/Coronavirus/THANZ-VIPIT-VITT-Advisory-statement.pdf(accessed on 7-4-2022).
- Dyer O. (2021). Covid-19: Regulators warn that rare Guillain-Barré cases may link to J&J and AstraZeneca vaccines. BMJ (Clinical research ed.), 374, n1786.
- Sadoff, J., Davis, K., & Douoguih, M. (2021). Thrombotic thrombocytopenia after Ad26.COV2.S vaccination— response from the manufacturer. N Engl J Med., 384, 1965-1966.
- Kwong, J.C., Vasa, P.P., Campitelli, M.A., Hawken, S., Wilson, K., et al. (2013). Risk of Guillain-Barre syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis, 13, 769-776.
- Kant, A., van Hunsel, F., & van Puijenbroek, E. (2021). Numbers of spontaneous reports: How to use and interpret?. British journal of clinical pharmacology, 10.1111/bcp.15024. Advance online publication.https://doi.org/10.1111/bcp.15024
- EMA. (2022). Good pharmacovigilance practices. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices(accessed on 5-4-2022).
