| Original Article, Biomed Biopharm Res., 2023; 20(2):43-57 doi: 10.19277/bbr.20.2.326; Bilingual PDF [+]; Portuguese html [PT] |
Development and evaluation of the effectiveness of a solid shampoo bar
Laryssa Silva de Lima 1*, Giovanna Calderaro Illiceto 1*, Maria Valéria Robles Velasco 2, & Michelli Ferrera Dario 1,2 ![]() ✉️
✉️
1 - University Center São Camilo, São Paulo, Brazil
2 - Department of Pharmacy, School of Pharmaceutical Sciences, University of São Paulo, São Paulo, Brazil
* These authors made an equal contribution to the development of this study.
Abstract
Solid shampoo is an innovative and alternative cosmetic form of shampoo that, as it does not contain water in its composition, gains the attribute of being more sustainable. This work aims to develop, evaluate the stability, and characterize a solid shampoo formulation. Nine solid shampoo formulations were prepared by varying the concentrations of solid surfactants defined through a statistical design. The formulations, labeled F1 to F9, were submitted to the preliminary stability evaluation under different storage conditions for 30 days, in which organoleptic characteristics as well as the pH values were evaluated. Approved formulations were physically and functionally characterized. Only two formulations (F3 and F5) were approved in the stability assessment because they did not show changes in consistency, color, odor, appearance, and pH under different storage conditions. These formulations showed stable foaming, but crumbled very easily and presented low hardness, which indicates the need to adjust the concentration of consistency agents to make the formulation more resistant to breakage. The formulations demonstrated excellent cleaning and conditioning ability.
Keywords: Stability, hair cosmetics, hair, shampoo, surfactants
To Cite: Silva de Lima, L., et al. (2023) Development and evaluation of the effectiveness of the solid shampoo bar. Biomedical and Biopharmaceutical Research, 20(2), 28-42
Author correspondence:
Received: 25/07/2023; Accepted: 07/12/2023
Introduction
Shampoos are products primarily intended to promote cleansing of hair and scalp (removal of oils, skin particles, dandruff, dirt, environmental pollutants and other contaminating particles). However, in addition to the elementary attributes, the formulation is expected to guarantee beautification and parameters such as hair combability and mechanical resistance (1).
Shampoo formulations are basically composed of a mixture of surfactants incorporated in a convenient cosmetic form: liquid, solid or powder. Surfactants are the main ingredients in shampoos because, in addition to their high cleaning capacity, they form foam (a quality desired by consumers) due to the amphiphilic characteristics of these molecules (2).
Shampoo bars are an alternative cosmetic form that, unlike conventional shampoo (liquid), does not contain water as the main ingredient in its formulation, making it more sustainable (3,4). Allied to the low amount of water, it is expected that the product needs a lower concentration of substances with preservative action, and this would have a positive impact on the environment since some molecules, such as parabens, can accumulate in marine and water animals sweet (5,6). Reducing the need for preservative ingredients is also interesting as it reduces the ability of the product to cause allergic contact dermatitis and other allergic reactions (7).
However, obtaining solid shampoo represents a challenge since most of the surfactants (cleaning agents and foam formers) currently available on the market have a liquid consistency. The problems frequently found in commercially available solid shampoos are hardness in the bar (plasticity), lack of shine, cracking, foam not pleasant in quantity and creaminess, roughness, and wear in use (loss of mass, softening of the bar) (8,9). Thus, developing and improving a solid shampoo formulation containing innovative ingredients while maintaining the desired attributes of this cosmetic class is essential. Therefore, this work aimed to develop, evaluate the preliminary stability and characterize physically solid shampoo bar formulations.
Materials and methods
Development and preparation of formulations
The quantitative composition of the formulations (Table 1) was obtained according to mixture-type statistical planning (DOE), in which the concentrations of the three solid surfactants used were varied (sodium cocoyl isethionate, sodium coco sulfate, and sodium lauryl sulfoacetate) as their sum corresponded to 100% of the surfactant mixture. Statistical planning was conducted using R software, resulting in nine formulations.
| Table 1 - Quali-quantitative composition of the formulations obtained by statistical mixture design (DOE). |
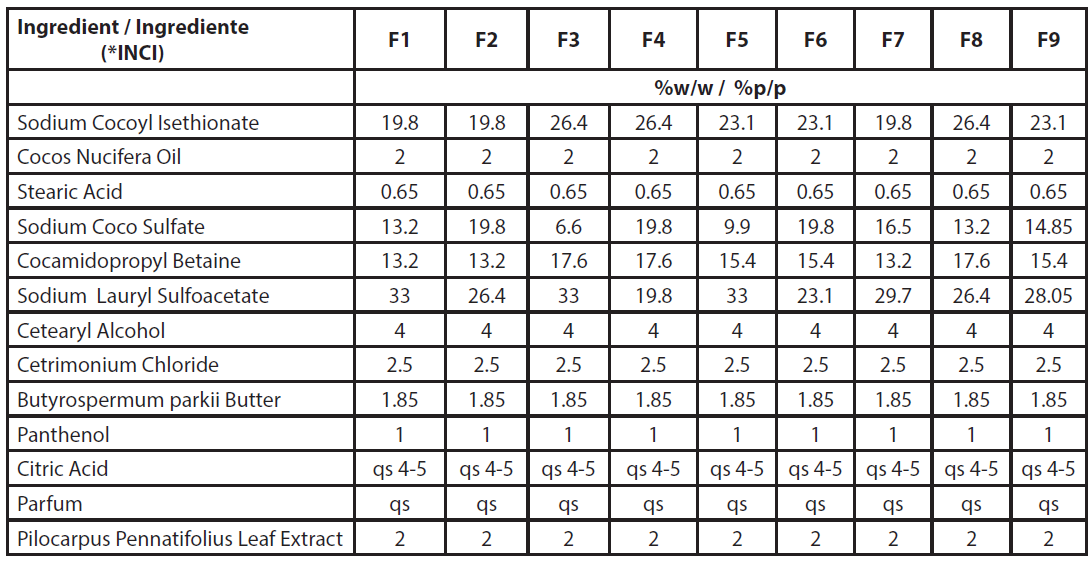 |
| *INCI: International Nomenclature of Cosmetic Ingredients |
The preparation of the formulations began by mixing sodium isethionate with cocoamidopropyl betaine, which remained under heating in a water bath for approximately one hour until a paste was formed, as shown in Figures 1a-d. This step was necessary because sodium isethionate flakes have a high melting range (191-194 ºC), which hinders its homogeneous incorporation only by heating. A previous mixture with cocoamidopropyl betaine facilitates the process. After the mixture was p repared under heating, the paste was crushed with the aid of a mortar and pestle to guarantee an improved homogenity of the mixture (Figure 1c).A mixture of sodium isethionate and cocoamidopropyl betaine. (a) The image shows the heating performed in a water bath. (b) Paste formation of sodium isethionate and cocoamidopropyl betaine. (c) Paste of sodium isethionate and cocoamidopropyl betaine being milled. (d) Paste after shredding.
| Figure 1 - A mixture of sodium isethionate and cocoamidopropyl betaine. (a) The image shows the heating performed in a water bath. (b) Paste formation of sodium isethionate and cocoamidopropyl betaine. (c) Paste of sodium isethionate and cocoamidopropyl betaine being milled. (d) Paste after shredding. |
 |
Subsequently, the other ingredients (except the Jaborandi glycolic extract) were weighed and incorporated into the sodium isethionate and cocoamidopropyl betaine paste under heating. After obtaining a homogeneous mixture, it was removed from heating, and the glycolic extract of Jaborandi was added under vigorous manual agitation. The formulation was transferred to appropriate molds and cooled in a refrigerator until solidification.
Preliminary Stability Evaluation
The formulations were subjected to stress conditions for 30 days to accelerate the appearance of possible signs of instability. The samples were stored under the following conditions: room temperature (22 ± 2 ºC), heating (40 ± 2 ºC), and cooling (5 ± 1 ºC). The samples were evaluated for organoleptic characteristics (appearance, color, and odor) and pH value at times 0 (zero), 7, 14, and 30 days (10). Time zero was considered around 48 hours after the preparation of the formulation.
The analysis of the organoleptic characteristics was conducted visually. The pH measurements were made with a pH meter using 10% (w/w) dispersions of solid shampoo formulations in purified water.
Foam Formation Test
The test used to characterize the formed foam was an adaptation of the Ross-Miles Test (11). About 1 gram of shampoo, previously triturated, was transferred to graduated cylinders. Deionized water was added up to the 50 ml level. The graduated cylinder was inverted three times. The foam level generated at the initial instant and after 20 minutes of rest was recorded. The test was performed in triplicate.
Crack test
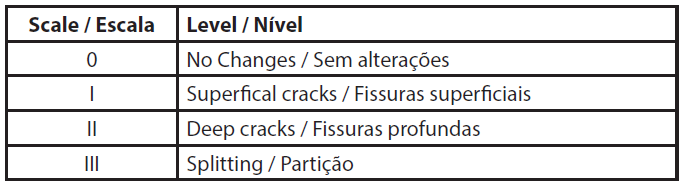
Shampoo samples (in triplicate) were suspended in a system where half of the shampoo was immersed in deionized water inside a plastic container for 24 hours at room temperature. After this period, they were removed from the water and remained suspended for drying for 32 hours. Subsequently, the levels of cracks were classified as 0, I, II, or III, according to Table 2 (8,12).
| Tabela 2 - Scale for evaluating cracks (8). |
 |
Texture test
The analysis of texture parameters (consistency, hardness, cohesiveness, and work of cohesion) of the shampoo bars was conducted in penetration compression mode, in the Stable Micro Systems Texture Analyzer® texturometer (Surrey, United Kingdom) using the SMS P/N probe, penetration depth of 8 mm, test speed of 1 mm/s and trigger force of 0.049 N. Eight repetitions were made for each shampoo bar (in duplicate) and tests were performed at room temperature (~25°C).
Hair Strand Cleaning Test
Strands of virgin natural straight Caucasian hair (triplicate per group), purchased commercially, with 15 cm in length and mass of 2.5 g, were initially weighed obtaining its exact initial mass. Subsequently, these were immersed in a solution of artificial sebum dispersed in hexane (6% w/w) After evaporation of the organic solvent, the resulting condition mimics the natural human sebum that adheres to the hair fiber, leaving it with a dirty appearance (13,14). The artificial sebum (Table 3) was prepared by simply mixing the ingredients after melting.
| Tabela 3 - Quali-quantitative composition of the artificial sebum. |
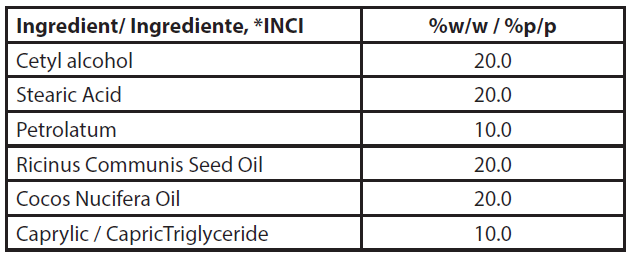 |
| *INCI: International Nomenclature of Cosmetic Ingredients |
Hair locks remained in contact with the artificial sebum solution for 20 minutes with the aid of a magnetic stirrer. Subsequently, the excess solution was removed manually and left to rest for 24 hours at room temperature (22.0 ± 2.0 ºC) and controlled relative humidity (65% RH), for drying. After this period, the locks were weighed again, obtaining the mass that comprises the sum of the lock of hair and the adhered sebum.
After drying, the locks were washed according to a standardized procedure. Initially, the locks were rinsed for 10 seconds in warm water at 40.0 ± 1.0 °C. Approximately 1.0 g of shampoo was then applied to the locks, followed by a massage for 1 minute. Subsequently, the locks were rinsed for 30 seconds with warm water at 40.0 ± 1.0ºC. Excess water was removed manually, and the locks were left to dry naturally at room temperature (22.0 ± 2.0°C) for at least 48 hours (15). After drying, the locks were weighed obtaining the sum of the mass of the lock and the residual artificial sebum. This assay was performed in triplicate. The detergency power was calculated by the percentage of sebum removed, according to Equation 1 (14).
| [Eq. 1] |
where: DP = detergency power; SR = amount of residual sebum present in the lock after the washing procedure; ST= amount of total sebum present in the lock before the washing procedure.
Combability
Total work for dry combing was determined on the Dia-Stron® MTT 175 (Dia-Stron LTD, Hampshire, UK)at room temperature (22.0 ± 2.0ºC) and 65% RH. The locks of hair (approximately 2.5 g in mass and 15 cm in length) were initially combed ten times using a plastic comb to untangle the strands, thus preventing damage to the equipment. Five trials, at least, were conducted for each lock, and between each trial, the lock was rotated 360º five times (16). The percentage change in force required for dry combing was calculated by comparing the values obtained for the same lock of hair, before and after washing with the solid shampoo. The test was done in triplicate.
Statistical analysis
Statistical analysis was performed using Student's t-test for two means, except the combing test, in which the paired t-test was used, comparing the results obtained before and after washing the locks with solid shampoo. The significance level adopted was α=0.05.
Results and discussion
Shampoo formulations are generally prepared from a mixture of anionic, amphoteric and cationic surfactants. The anionic substances used in this work (sodium coco sulfate, sodium cocoyl isethionate and sodium lauryl sulfoacetate) have a high cleaning and foaming capacity. Sulfoacetates (e.g., sodium lauryl sulfoacetate) and sulfosuccinates (e.g., disodium lauryl sulfosuccinate) can be used for milder “Sodium Lauryl Ether Sulfate (LESS)-free” shampoos. Isethionates have been used to obtain synthetic bars as they are gentle on the skin and have recently attracted much interest as suitable replacement for sulfates in “sulfate-free” shampoos. Cocoamidopropyl betaine is one of the amphoteric secondary surfactants most used in shampoos, it has a low irritation potential compared to sulfated ones but reduces the foaming capacity of anionic compounds (17). Cationic surfactants, such as cetrimonium chloride, act as conditioning agents, improving combability and neutralizing the static negative charge present on the surface of the hair shaft (18).
The formulations, prepared from the experimental design, had a characteristic coconut aroma, due to the various raw materials derived from coconut present in it (cocoyl sodium isethionate, coconut oil, and sodium coco sulfate), white color, and homogeneous appearance (Figure 2).
| Figure 2 - Example of the appearance of the solid shampoo. |
 |
According to the Stability Guide for Cosmetic Products of ANVISA (Brazil) (10), the Preliminary Stability Evaluation aims to guide the choice of formulations in the product development stage. Thus, due to the nature of the test, the formulations are exposed to extreme temperature conditions, which allows the anticipation of possible reactions between the components, being useful in the screening stage. Changes in the pH value, for example, may indicate the occurrence of some chemical reaction, including degradation of the present components and oxidation (19). Information on organoleptic characteristics (appearance, odor, and color) is also essential, as they show chemical and physical changes in the inputs used.
The pH values of the formulations during the stability test under storage conditions at room temperature (22 ± 2 ºC), heating in an oven (40 ± 2 ºC), and cooling in a refrigerator (5 ± 1 ºC) reached the maximum value of 6.6, independent of temperature. The surfactant sodium cocoyl isethionate is easily hydrolysable, generating fatty acids and sodium isethionate, a substance that, in water, has a pH of 7 to 11. Therefore, the increase in pH observed may be the result of the formation of sodium isethionate during storage of the formulation in the presence of moisture (20). The pH values are considered acceptable because the hair fiber, like the skin, has a slightly acidic pH, being biocompatible with pH values between 4.5 and 7.0 (21).
Regarding the aspect of the formulations, at room temperature storage conditions (22.0 ± 2.0 ºC), only formulations F3, F5, F7, and F8 did not show changes in consistency (solid), color (white), odor (characteristic of coconut), and aspect (homogeneous), being considered stable. The other formulations showed changes in appearance as the surfaces because they became heterogeneous. At high-temperature storage conditions (40.0 2.0 ºC), only formulations F3, F4, and F5 maintained their initial characteristics after 30 days of testing. Formulations F2, F6, F7, F8, and F9 showed a change in appearance (they had a heterogeneous surface), while F1 also showed a change in consistency, becoming semi-solid (pastey). When stored in a refrigerator (5.0 ± 1.0 ºC), only F1 did not maintain its initial characteristics after 30 days of testing. The greater number of unaltered formulations observed in low-temperature conditions can be explained by the lower speed of chemical reactions in this condition.
As only formulations F3 and F5 were stable at all storage temperatures for 30 days, they were physically and functionally characterized. Both formulations had the lowest concentrations of sodium coco surfactant sulfate and high proportions of sodium cocoyl isethionate and sodium lauryl sulfoacetate.
In general, the formulations approved in the stability tests performed well in foaming, with a small variation in volume during rest time. Formulation F3 generated 25.0 ± 4.0 ml of foam initially, while F5 generated 18.67 ± 2.31 ml. After 20 minutes of rest, the F3 presented a variation of 4.07 ± 0.66% and the F5 a variation of 2.08 ± 3.61%. Thus, the foam stability of the F3 formulation was 95.9 ± 0.7% while the F5 was 97.9 ± 3.6%. However, statistical analysis revealed that the samples did not show significantly different results (p = 0.447).
These results indicate that the prepared solid shampoo formulations have similar or greater foam stability values than liquid shampoo formulations, bar soaps, or liquid surfactant dispersions (22-25). Antonic et al. (22) evaluated bar soaps obtained by saponification reactions and observed that soaps prepared with canola or palm oil generated soaps with foam stability of around 70%. A dispersion of saponins extracted from Furcraea foetida showed a stability of 85% (24). Badi and Khan showed that the liquid shampoo formulations evaluated had foam stability of around 100% (25).
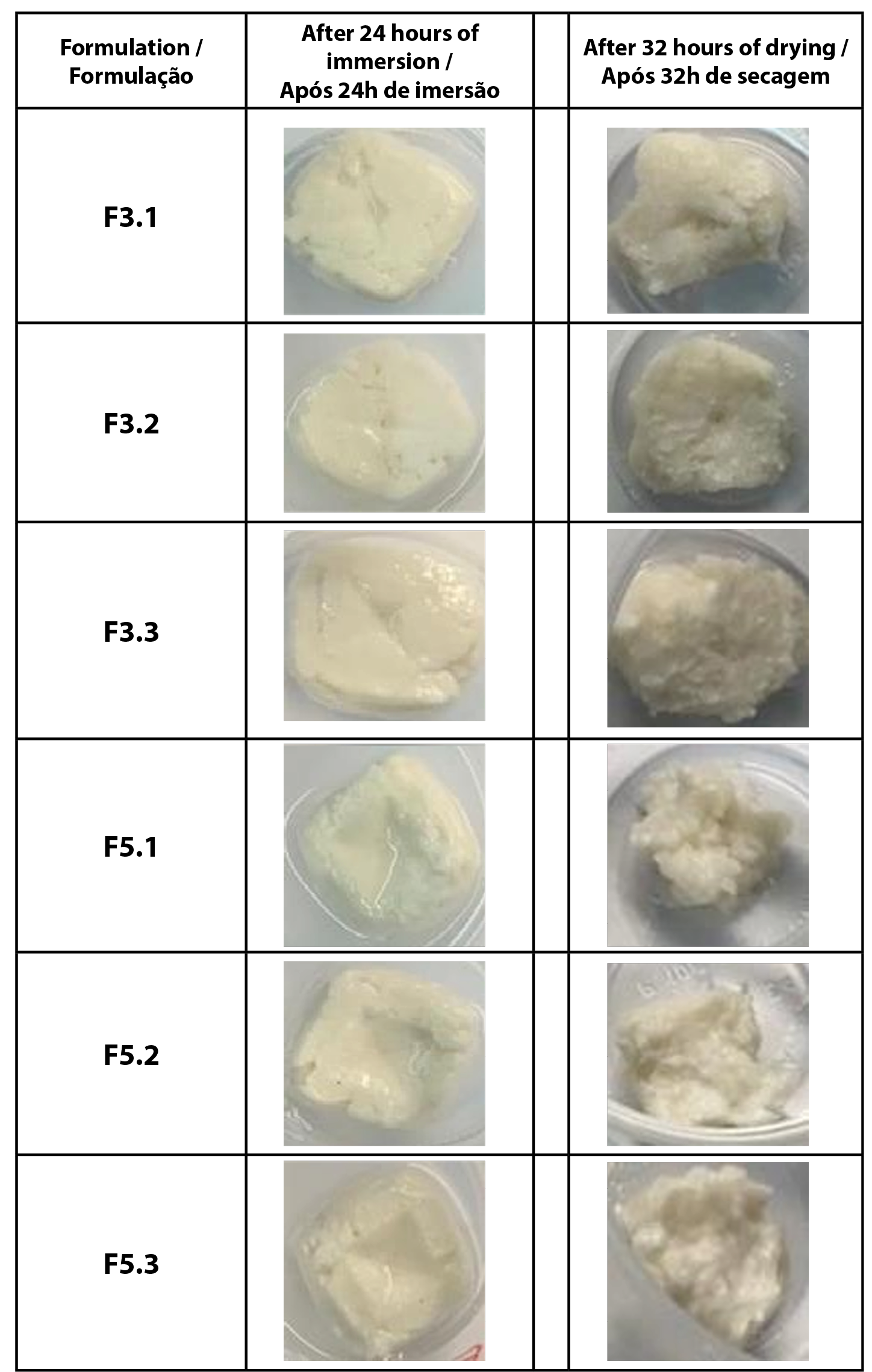
Cracking test results are intended to classify shampoos into cracking levels from 0 to III. Level 0 bars do not have cracks, Level I bars have cracks with superficial cracks, Level II bars have cracks with deep cracks, and Level III bars have deeper cracks, causing partitioning (26). As can be seen in Figure 3, it was not possible to evaluate the formation of cracks because the formulations collapsed soon after being removed from immersion. This result indicates the need to increase the concentration of consistency agents, namely stearic acid and cetostearyl alcohol, as it is known that incorporating consistency agents can reduce cracking in the formulation and increase its resistance.
| Figure 3 - The aspect of the solid shampoos in the Crack Test, indicated by formulation code and triplicate. |
 |
Texture characterization methods for cleaning bars involve penetration tests using texturometers and probes such as needles, cones, or wires. Thus, it is possible to measure parameters such as consistency, hardness, cohesiveness, and cohesion work through analysis of curves generated by the equipment that relate time and force (N) (27) (Figure 4). The peak or maximum force is considered the measure of firmness or hardness of the sample, that is, the higher the value, the firmer the sample. The area under the curve in this positive region of the graph indicates the consistency of the formulation. The maximum force observed in the negative portion of the graph, produced in the return of the probe, is related to the cohesiveness of the sample, while the area under the curve in the negative region indicates the work of cohesion (28). In this work, a needle-type probe was used, and the texture analysis results obtained for samples F3 and F5 are presented in Table 4.
| Figure 4 - Curve displayed in the texture analysis test. |
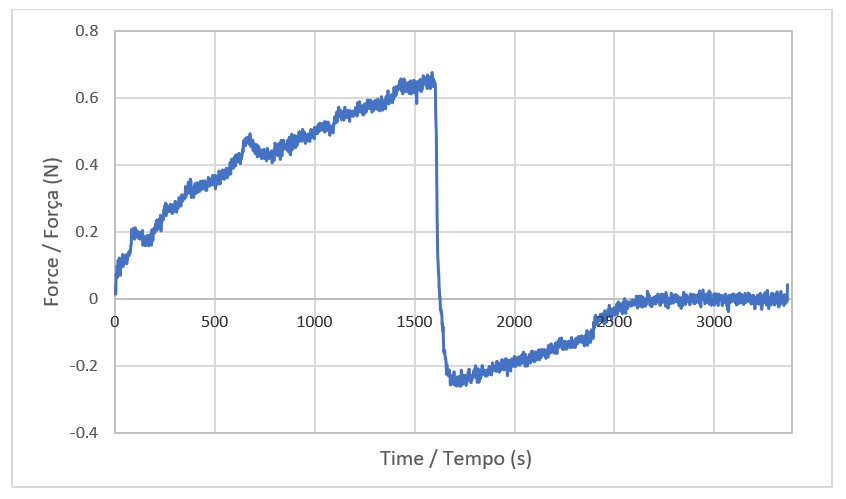 |
| Tabela 4 - Physical characterization of solid shampoo bar samples. |
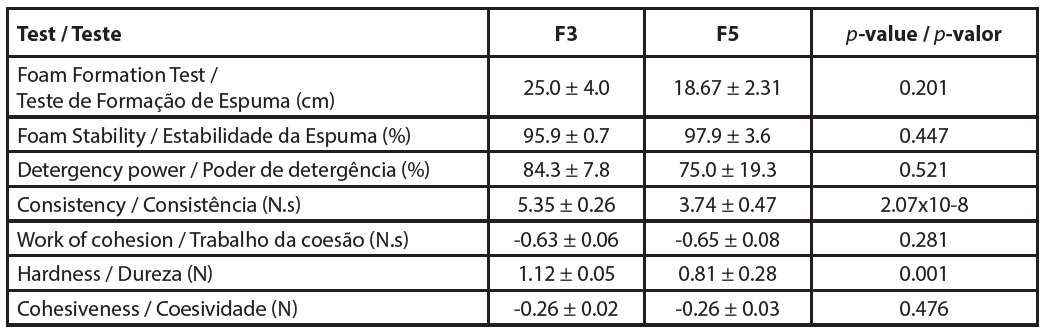 |
There are few works published in the literature that present texture analysis results of bar formulations, such as soaps or shampoos. Existing works more frequently present only firmness data, to the detriment of other tests. As there is no international standardization of the test parameters (probe type, penetration depth, test speed), the results obtained in this work cannot be compared with many others. Sousa (2020) developed bar soaps and obtained firmness results, using the same test parameters, in the order of 40N (26). Therefore, the shampoo bar (F3 and F5) showed low hardness values, 1.12 ± 0.05N e 0.81 ± 0.28N, respectively, which corroborates the results obtained in the crack test. From the small number of samples evaluated (only two were approved in the preliminary stability evaluation) and published works in this area, it is not possible to infer the impact of each surfactant on texture parameters. Therefore, new experiments are necessary to deepen understanding of this type of formulation.
Both formulations analyzed showed excellent potential for cleaning hair strands (around 80%), as shown in Table 4, in comparison with literature data that show that commercial liquid shampoo formulations have a cleaning capacity of around 60% (29). Statistical analysis revealed that the samples did not show significantly different results (p-value = 0.521), that is, the difference in composition did not impact this effectiveness parameter of the solid shampoo.
In the combability test, the statistical analysis revealed that both F3 and F5 did not impact the combability of the hair strands (Figure 5), since the total work values, before and after shampoo application, were not significantly different (p-value equal to 0.142 and 0.672, respectively), that is, the difference in composition does not influence this characteristic. However, notably, it is possible to observe a tendency towards a decrease in the conditioning power of the F3 formulation.
| Figure 5 - Total work to comb the locks before and after the washing step. |
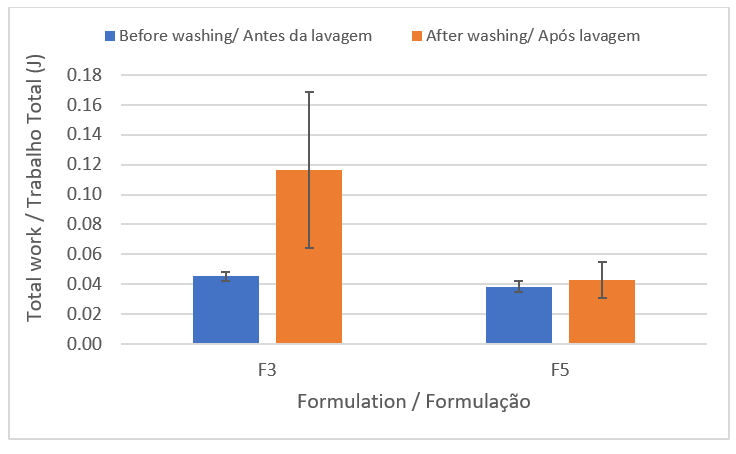 |
Despite the high concentration of anionic surfactants (sodium cocoyl isethionate, sodium coco sulfate, sodium lauryl sulfoacetate) present in the solid shampoo formulation, there was no increase in the force required for combing. This result is due to the presence of positively charged molecules, such as cocoamidopropyl betaine (amphoteric surfactant that has a net positive charge at the pH of the formulation) and cetyltrimethylammonium chloride (antistatic cationic agent). Both contribute to the neutralization of negative charges naturally present in the hair fiber as well as those deposited by the anionic surfactants in the formulation, thus reducing frizz and combing work (30). In addition, the formulations have moisturizing and emollient agents such as coconut oil, d-panthenol, and shea butter that, by lubricating the hair fiber, improve its combability (31).
Conclusions
Formulations approved in the preliminary stability evaluation showed stable foaming, and excellent cleaning and conditioning ability but had low hardness, which indicates the need to adjust the concentration of consistency agents to make the shampoo bars more resistant to breakage.
Acknowledgments
The authors would like to thank BASF and Clariant for the donation of raw materials.
Authors Contributions Statement
MFD, MVRB, conceptualization and study design; LLL, GCI, experimental implementation; LLL, GCI, data analysis; LLL, GCI, drafting, editing, and reviewing; LLL, GCI, tables, and figures; MFD, supervision, and final writing.
Conflict of Interests
The authors declare there are no financial and/or personal relationships that could present a potential conflict of interests.
References
1. Barel, A., Paye, M., & Maibach, H. (2014). Handbook of Cosmetic Science and Technology (4th ed.). CRC Press Taylor& Francis Group.
2. Krunali, T., Dhara, P., Meshram, D. B., & Mitesh, P. (2013). Evaluation of standards of some selected shampoo preparation. World Journal of Pharmacy and Pharmaceutical Sciences, 2(5), 3622–3630.
3. Gubitosa, J., Rizzi, V., Fini, P., & Cosma, P. (2019). Hair Care Cosmetics: From Traditional Shampoo to Solid Clay and Herbal Shampoo. A Review. Cosmetics, 6(1), 13. https://doi.org/10.3390/cosmetics6010013
4. Brito, I., Ferreira, S. M., & Santos, L. (2023). On the Path to Sustainable Cosmetics: Development of a Value-Added Formulation of Solid Shampoo Incorporating Mango Peel Extract. Cosmetics, 10(5), 140. https://doi.org/10.3390/cosmetics10050140
5. Jeong, Y., Xue, J., Park, K. J., Kannan, K., & Moon, H.-B. (2019). Tissue-Specific Accumulation and Body Burden of Parabens and Their Metabolites in Small Cetaceans. Environmental Science & Technology, 53(1), 475–481.
6. Marta-Sanchez, A. V., Caldas, S. S., Schneider, A., Cardoso, S. M. V. S., & Primel, E. G. (2018). Trace analysis of parabens preservatives in drinking water treatment sludge, treated, and mineral water samples. Environmental Science and Pollution Research, 25(15), 14460–14470. https://doi.org/10.1007/s11356-018-1583-4
7. Voller, L. M., & Warshaw, E. M. (2021). Killing Two Birds With One Bar: Shampoo and Conditioner Bars Are Low Allergen and Environmentally Friendly. Dermatitis : contact, atopic, occupational, drug, 32(4), e60–e64. https://doi.org/10.1097/DER.0000000000000627
8. Sasson, C., Boin, G., Cararo, G., Cordeiro, M., Dusi, L., & Nunes, P. (2009). Influência de Emolientes em Sabonetes em Barra. Cosmetics & Toiletries Brasil, 21(3), 50–60.
9. Friedman, M. (2016). Chemistry, Formulation, and Performance of Syndet and Combo Bars. In Soap Manufacturing Technology (pp. 73–106). Elsevier. https://doi.org/10.1016/B978-1-63067-065-8.50004-9
10. Brasil. (2004). Guia de Estabilidade de Produtos Cosméticos (1st ed.). ANVISA.
11. Klein, K. (2004). Evaluating Shampoo Foam. Cosmetic &Toiletries, 119(10), 32–35.
12. Diez, A. M., & Carvalho, C. S. G. (2000). Aditivos para sabonetes em barra. Oxiteno S/A Indústria e Comércio. http://www.oxiteno.com.br/aplicacoes/mercados/doc/documento.asp?artigotecnico=9
13. Gour, V. S., Sanadhya, N., Sharma, P., Parmar, A., & Datta, M. (2015). Biosurfactant characterization and its potential to remove sebum from hair. Industrial Crops and Products, 69, 462–465. https://doi.org/10.1016/j.indcrop.2015.03.007
14. Chen, Y.-F., Yang, C.-H., Chang, M.-S., Ciou, Y.-P., & Huang, Y.-C. (2010). Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. International Journal of Molecular Sciences, 11(11), 4417–4425. https://doi.org/10.3390/ijms11114417
15. Dario, M. F., Pahl, R., Castro, J. R., Lima, F. S., Kaneko, T. M., Pinto, C. A. S. O., Baby, A. R., & Velasco, M. V. R. (2013). Efficacy of Punica granatum L.hydroalcoholic extract on properties of dyed hair exposed to UVA radiation. Journal of Photochemistry and Photobiology B: Biology, 120, 142–147.
16. Evans, A., Marsh, J., & Wickett, R. (2011). The structural implications of water hardness metal uptake by human hair. International Journal of Cosmetic Science, 33(5), 477–482.https://doi.org/10.1111/j.1468-2494.2011.00659.x
17. Cornwell, P. A. (2018). A review of shampoo surfactant technology: consumer benefits, raw materials and recent developments. International Journal of Cosmetic Science, 40(1), 16–30. https://doi.org/10.1111/ics.12439
18. Johansson, I., & Somasundaran, P. (2007). Shampoo Formulation. In K. Klein & I. Palefsky (Eds.), Handbook for Cleaning/Decontamination of Surfaces (pp. 277–304). Elsevier.
19. Taylor, K. M. G., & Aulton, M. E. (2022). Aulton’s Pharmaceutics: The Design and Manufacture of Medicines (6th ed.). Elsevier Health Sciences.
20. Sakai, T. (2017). Body Care Cosmetics. In Cosmetic Science and Technology (pp. 561–570). Elsevier. https://doi.org/10.1016/B978-0-12-802005-0.00033-1
21. Halal, J. (2009). Hair structure and chemistry simplified (5th ed.). Cengage Learning.
22. Antonic, B., Dordevic, D., Jancikova, S., Tremlova, B., Nejezchlebova, M., Goldová, K., & Treml, J. (2021). Reused Plant Fried Oil: A Case Study with Home-Made Soaps. Processes, 9(3), 529. https://doi.org/10.3390/pr9030529
23. Rajput, G., Janni, D. S., Subramanyam, G., Ray, D., Aswal, V., & Varade, D. (2022). Novel approach for tuning micellar characteristics and rheology of a sulfate-free anionic surfactant sodium cocoyl glycinate. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 648, 129426. https://doi.org/10.1016/j.colsurfa.2022.129426
24. Randriamamonjy, T. H., Ontiveros, J. F., Andrianjafy, M. T., Samiez, P., Berlioz-Barbier, A., Nardello-Rataj, V., Aubry, J.-M., Ramanandraibe, V., & Lemaire, M. (2022). Comparative study on the amphiphilicity, emulsifying and foaming properties of saponins extracted from Furcraea foetida. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 653, 129923. https://doi.org/10.1016/j.colsurfa.2022.129923
25. Al Badi, K., & Khan, S. A. (2014). Formulation, evaluation and comparison of the herbal shampoo with the commercial shampoos. Beni-Suef University Journal of Basic and Applied Sciences, 3(4), 301–305. https://doi.org/10.1016/j.bjbas.2014.11.005
26. Sousa, L. L. de C. (2020). Estudo da estabilidade físico-química do sabonete em barra contendo o álcool polivinílico (PVA) [Trabalho de Conclusão de Curso]. Universidade Federal de Uberlândia.
27. Yarovoy, Y., & Post, A. J. (2016). Soap Bar Performance Evaluation Methods. In Soap Manufacturing Technology (pp. 247–266). Elsevier. https://doi.org/10.1016/B978-1-63067-065-8.50011-6
28. Tai, A., Bianchini, R., & Jachowicz, J. (2014). Texture analysis of cosmetic/pharmaceutical raw materials and formulations. International Journal of Cosmetic Science, 36(4), 291–304. https://doi.org/10.1111/ics.12125
29. Moghimipour, E., Jasemnezhad, M., Mohammad Soleymani, S., & Salimi, A. (2021). Preparation and evaluation of a free surfactant herbal shampoo with Acanthophyllum Squarrosum Saponins. Journal of Cosmetic Dermatology, 20(1), 181–187. https://doi.org/10.1111/jocd.13483
30. Fernández-Peña, L., & Guzmán, E. (2020). Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics, 7(2), 26. https://doi.org/10.3390/cosmetics7020026
31. Dabbur, F. S., Lima, G. H. da S., Costa, R. M., Costa, C. L., Santos, L. E. S., Luz, V. B., de Vasconcelos, C. C., & Costa, J. R. de M. (2019). Development, physicochemical and functional analysis of anti-frizz leave-on emulsion with coconut oil. International Journal of Phytocosmetics and Natural Ingredients, 6(1), 8–8. https://doi.org/10.15171/ijpni.2019.08
